Am Fam Physician. 2014;90(9):625-631
Author disclosure: No relevant financial affiliations.
Early detection of cancer is a core task in family medicine, and patients have come to expect screening tests, sometimes out of proportion to what evidence can justify. To understand the controversies surrounding screening and to provide sound advice to patients, family physicians should be familiar with the fundamental concepts of screening. Failure to account for the effects of lead-time, length-time, and overdiagnosis biases can lead to overestimation of screening benefits. For this reason, the best method for evaluating the benefit of screening tests is a randomized controlled trial showing decreased disease-specific or all-cause mortality. The number needed to screen can be used to measure the magnitude of benefit of screening tests. Accepted screening tests often require screening several hundred to more than 1,000 asymptomatic patients to prevent one death from the disease. The U.S. Preventive Services Task Force and American Academy of Family Physicians recommend screening for colorectal cancer in adults 50 to 75 years of age, and recommend against prostate-specific antigen testing to screen for prostate cancer. Annual low-dose computed tomography screening for lung cancer reduces mortality in persons 55 to 80 years of age with at least a 30-pack-year history who are otherwise healthy smokers or who have quit smoking within the past 15 years; however, it is associated with a high false-positive rate, uncertain harms from radiation exposure, and overdiagnosis. Therefore, it should be performed only in conjunction with smoking cessation interventions.
Cancer screening is an essential part of family medicine, and payers increasingly use compliance with screening guidelines to evaluate quality of care. Patients have come to expect screening tests, sometimes out of proportion to what evidence can justify. Changing recommendations for cancer screening have led to widespread media coverage and confusion among physicians and patients. To help patients navigate the controversies regarding screening, family physicians need to understand key concepts of screening, current recommendations, and possible future controversies.
| Recommendations from the USPSTF | SORT evidence rating | References | Comments |
|---|---|---|---|
| The USPSTF recommends colorectal cancer screening in patients 50 to 75 years of age using colonoscopy every 10 years, fecal occult blood testing every year, or flexible sigmoidoscopy every five years plus fecal occult blood testing every three years. Computed tomography colonography is not recommended for routine screening. | A | 10 | The AAFP concurs.11 |
| The USPSTF recommends against routine prostate-specific antigen testing to screen for prostate cancer. | B | 21 | The American Cancer Society and American Urological Association support shared decision making in men 55 to 69 years of age.23,24 |
| The USPSTF recommends annual low-dose computed tomography to screen for lung cancer in persons 55 to 80 years of age with at least a 30-pack-year smoking history who are otherwise healthy smokers or who have quit smoking within the past 15 years. | B | 30 | The AAFP found insufficient evidence to recommend for or against low-dose computed tomography screening.33 |
Key Issues
Screening can be defined as the application of diagnostic tests in asymptomatic patients for the purpose of dividing them into two groups: those who have a condition that would benefit from early intervention and those who do not.1 Screening is performed to detect disease at an early, asymptomatic stage when it is potentially more amenable to treatment. However, advancing the time of diagnosis alone does not justify screening; early diagnosis must also lead to measurable improvements in morbidity and mortality compared with clinical detection of symptomatic disease.2 An ideal screening program has the following characteristics3:
Characteristics of the disease: significant effect on quality or length of life, prevalence is high enough to justify costs, effective and acceptable treatment available, asymptomatic period during which detection and treatment significantly reduce morbidity and mortality
Characteristics of the test: sensitive enough to detect disease during the asymptomatic period, specific enough to minimize false positives, acceptable to patients
Characteristics of the population screened: sufficiently high disease prevalence, accessibility of follow-up medical care, willingness to comply with subsequent diagnostic tests and necessary therapy.
Although the rationale for screening seems compelling, there can be good reasons not to screen. Any given individual has a low likelihood of benefiting from a screening test, and the harms of screening will accrue to otherwise healthy patients. Because the prevalence of the screened disease in an asymptomatic population is usually low, most positive screening results will be false positives (i.e., low positive predictive value). False-positive results can lead to anxiety and the cost of additional work-up. Other potential harms of screening include false-negative results, which lead to false reassurance, and overdiagnosis (i.e., detection of disease that will never cause symptoms). Finally, when treatment is ineffective or has important harms, early detection can result in net harm rather than benefit. Compared with older guidelines, recent guidelines are more likely to consider both the harms and benefits of screening.
Bias in the Evaluation of Screening Tests
Figure 1 demonstrates that cancer is heterogeneous, existing along a spectrum ranging from indolent tumors that can arise and even regress without being detected to highly aggressive tumors that are likely to metastasize before they can be detected by screening. Only those tumors that grow slowly enough to be detected during the screening interval but are still capable of metastasizing are amenable to screening. All types of cancer exhibit all of these subtypes (from indolent to aggressive) and, for most types of cancers, only a minority of tumors are amenable to screening. This leads to a paradox: most fatal tumors are not detected by screening, whereas many tumors that are detected by screening have a low risk of metastasis, leading to the problem of overdiagnosis.
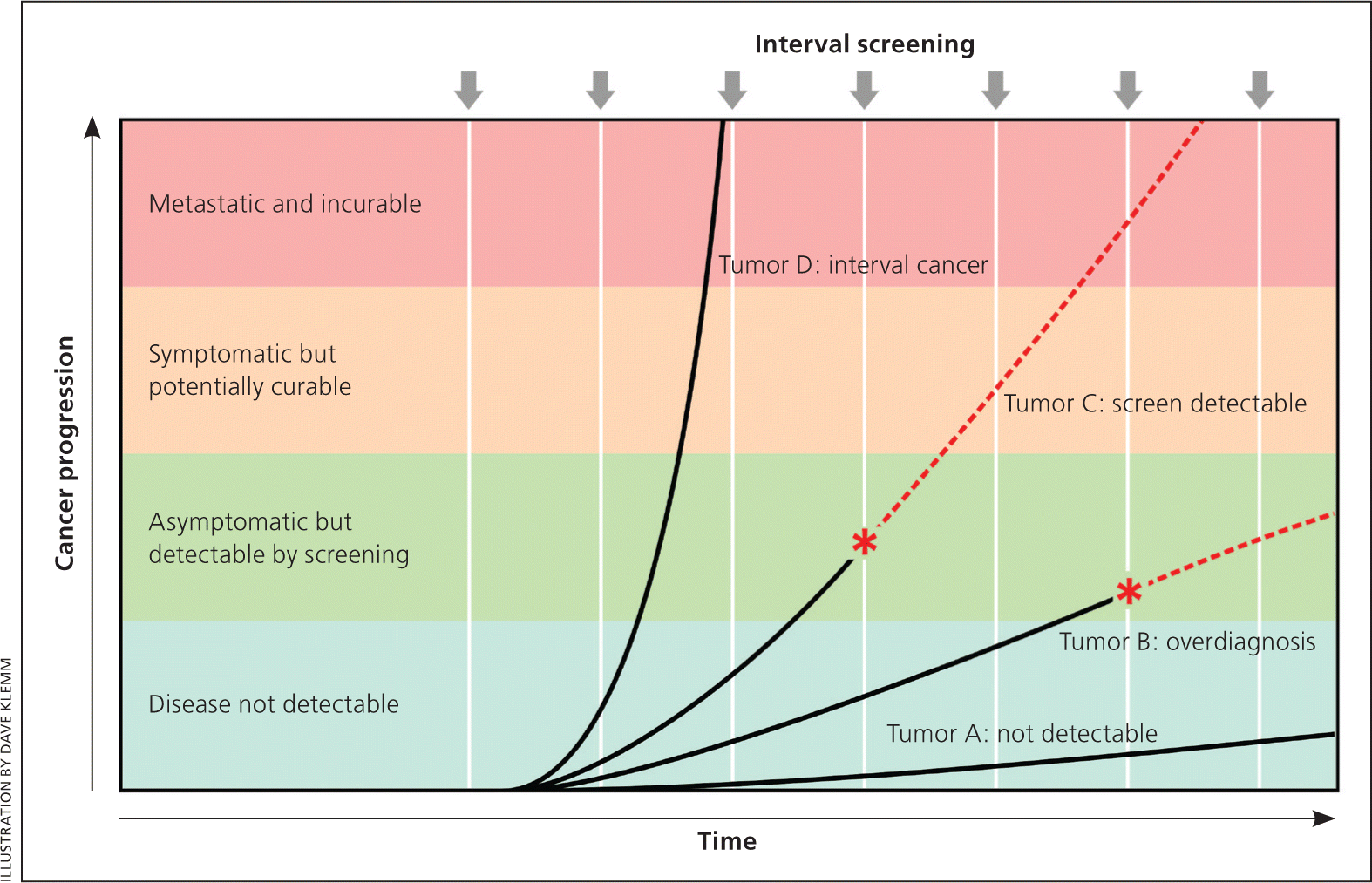
Bias arises when clinicians fail to take into account the natural history of asymptomatic disease, which can result in a systematic overestimation of screening benefit. Lead-time bias (Figure 24 ), length-time bias, (Figure 34 ), and overdiagnosis bias (Figure 44 ) are best understood schematically. In each of these examples, surrogate measures such as five-year survival or median survival time will appear to favor screening even in the absence of any effective treatment, but actual mortality is unchanged. Screening bias (or “healthy volunteer” bias) refers to the tendency of those who volunteer for screening (or in randomized trials, those who comply with screening protocols) to be healthier than those who do not; therefore, any apparent benefit may come solely from the self-selection of a healthy cohort.5
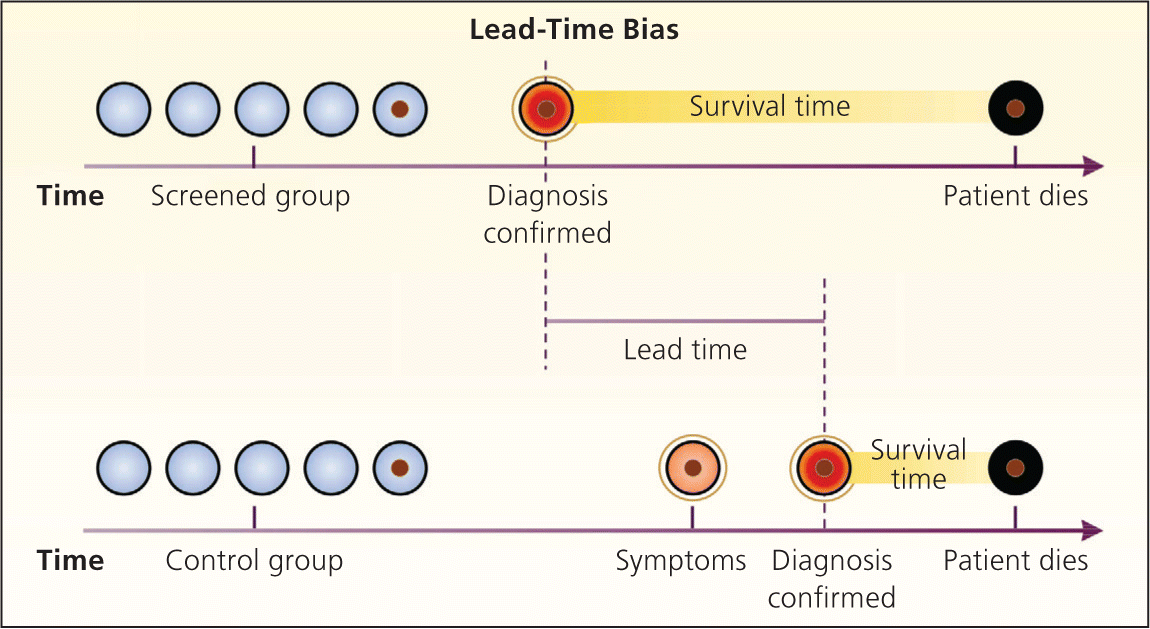
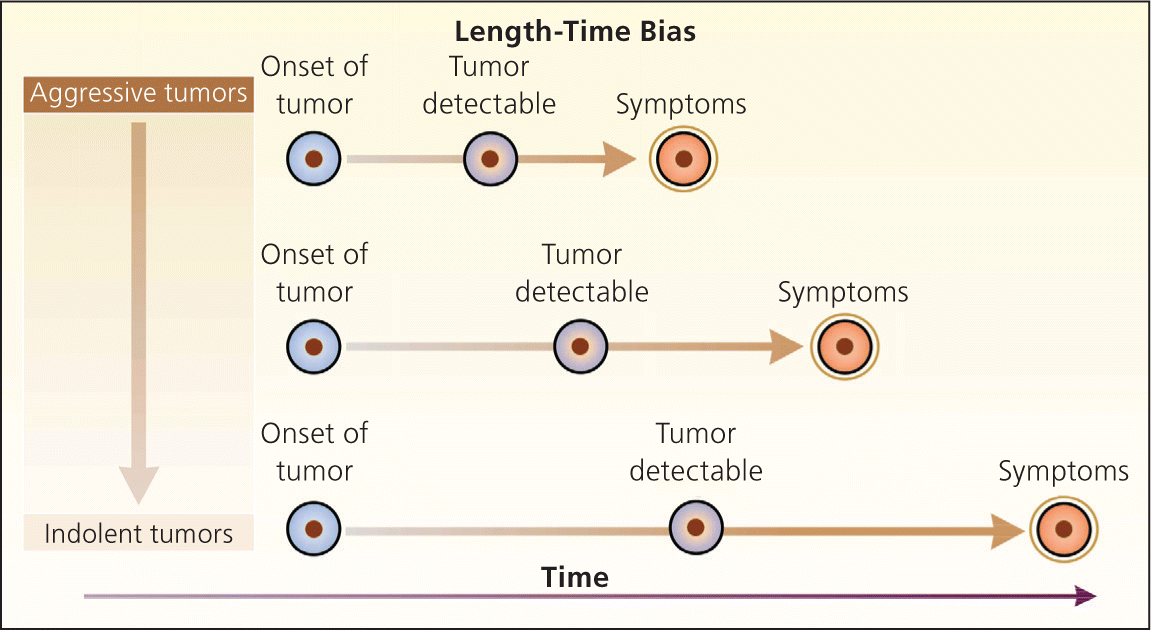
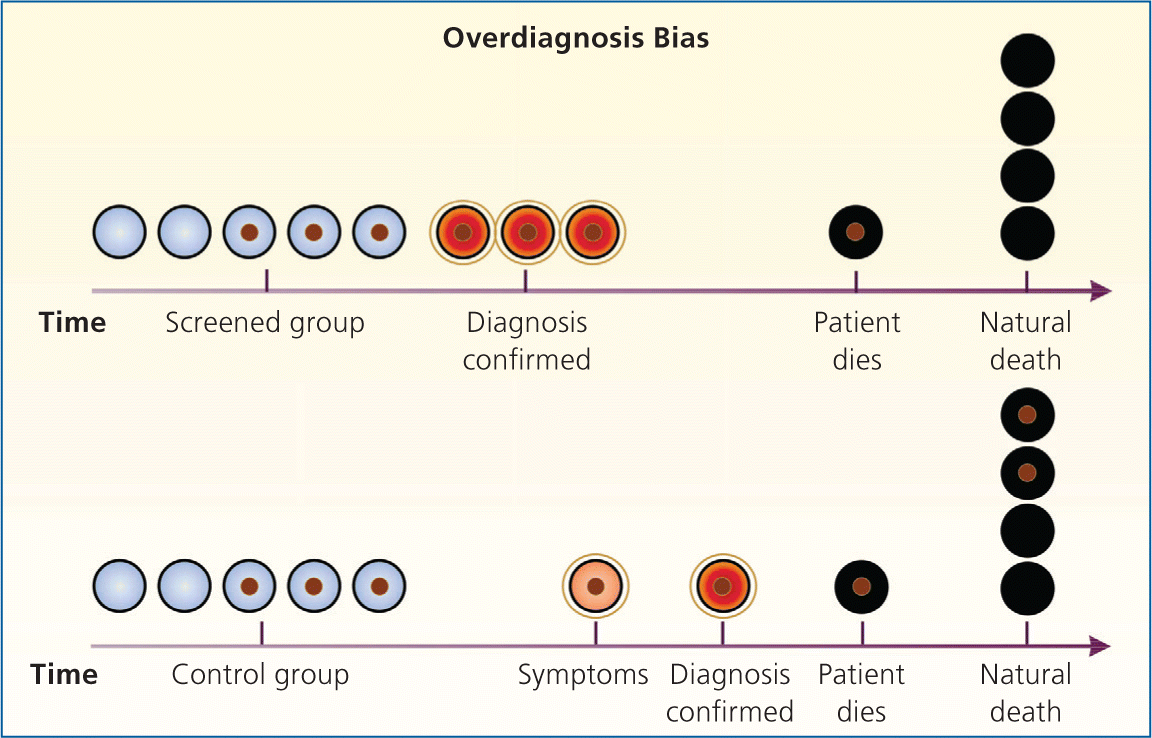
Because of the unpredictable nature of these biases, the most reliable evidence for the effectiveness of a screening test is a randomized controlled trial (RCT) of screening vs. no screening that uses intention-to-treat analysis, with disease-specific or all-cause mortality as the primary outcome. Because of low disease prevalence and long lead times, such trials can be lengthy, logistically difficult, and costly. In a screening trial showing a benefit, the absolute risk reduction in mortality in the screened population can be used to calculate the number needed to screen, or the number of patients who would need to be screened over a given time to prevent one death from the disease.2 Accepted screening tests often require screening several hundred to more than 1,000 asymptomatic patients to prevent one death from the disease.
Cancer Epidemiology
Cancer is the second leading cause of death in the United States and is the leading cause of death in those younger than 85 years.6 Age-adjusted mortality rates for cancer peaked in the early 1990s and have since declined by about 20%; they continue to decline at a rate of at least 1.5% per year. Four cancers account for nearly one-half of all cancer deaths, although mortality rates for these cancers have been declining: lung (27%), colorectal (9%), breast (14% in women), and prostate (10% in men).7
Screening Recommendations
COLORECTAL CANCER
The U.S. Preventive Services Task Force (USPSTF) and the American Academy of Family Physicians (AAFP) recommend screening for colorectal cancer in adults 50 to 75 years of age with colonoscopy every 10 years, fecal occult blood testing every year, or flexible sigmoidoscopy every five years plus fecal occult blood testing every three years.10,11 However, the Centers for Disease Control and Prevention estimates that in 2012, 27.2% of eligible adults had never been screened, and another 7.2% were not up to date with recommended screening tests. Colonoscopy was the most commonly used test (61.7%), followed by fecal occult blood testing (10.4%).12
In a Cochrane review of four randomized trials, three trials of fecal occult blood testing every two years showed a relative risk (RR) of 0.84 for colorectal cancer mortality in those randomized to screening and of 0.75 in those who actually underwent at least one screening test. The one study using annual screening showed an RR of 0.67 for those randomized to screening.13
A Cochrane review14 and a more recent systematic review15 of endoscopic screening for colorectal cancer showed an RR of 0.68 for colorectal cancer mortality with flexible sigmoidoscopy (four RCTs). Although results of ongoing RCTs of screening colonoscopy are not expected until after 2020, observational and case-control studies suggest an RR of 0.32 for colorectal cancer mortality with screening colonoscopy.15
Although colonoscopy is the most widely used test for colorectal cancer screening in the United States, it is also the most expensive and invasive test. Going forward, important questions will be whether colonoscopy will continue to be the dominant screening strategy, whether there is still a role for flexible sigmoidoscopy,16 and whether a noninvasive screening test might identify a high-risk subset most likely to benefit from colonoscopy. Noninvasive tests include computed tomography (CT) colonography, fecal immunochemical testing, and multitarget stool DNA testing. A recent study showed stool DNA testing to be significantly more sensitive, but less specific, for colorectal cancer and advanced precancerous lesions than fecal immunochemical testing.17
PROSTATE CANCER
Based on the results of two large RCTs and a Cochrane review,18–20 the USPSTF21 and AAFP22 recommend against routine prostate-specific antigen (PSA) testing to screen for prostate cancer. The American Cancer Society 23 and American Urological Association24 have revised earlier endorsements of PSA screening, and now advise against routine screening, instead advocating shared decision making based on patient values and preferences in men 55 to 69 years of age.
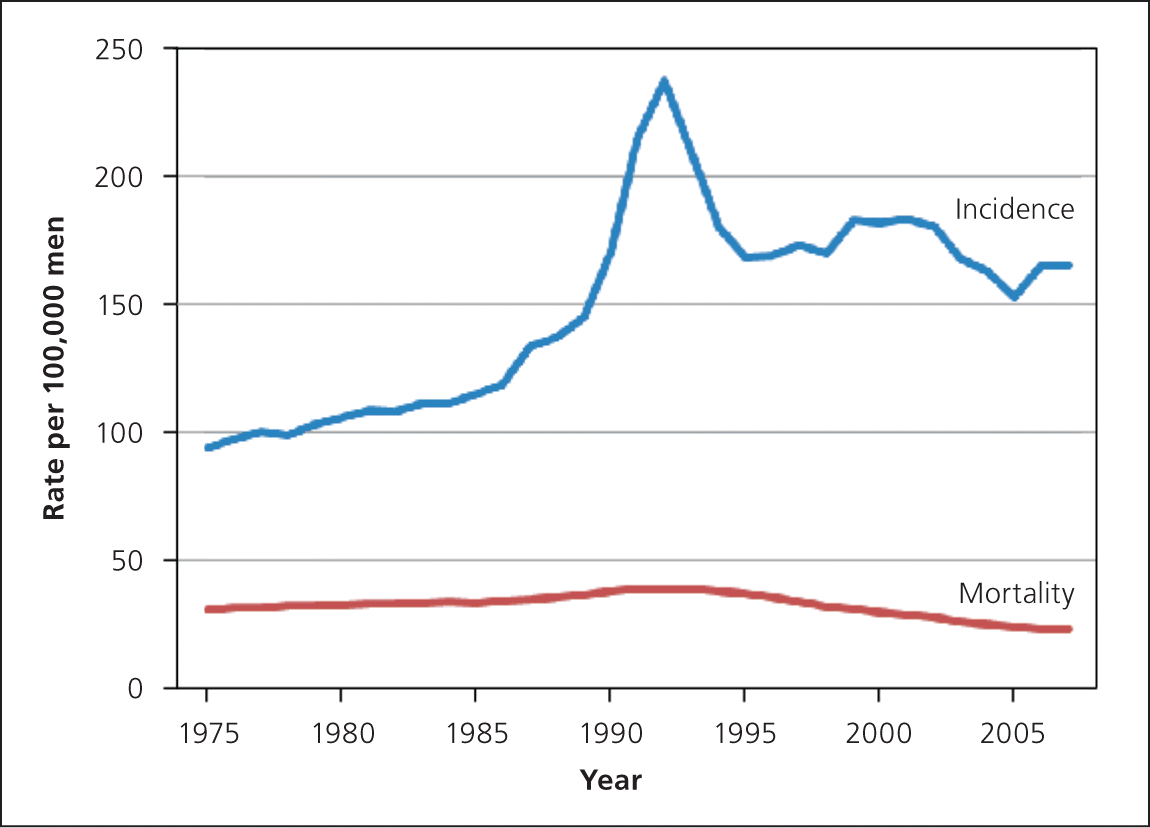
With the introduction of PSA testing in the late 1980s, the incidence of prostate cancer increased dramatically while mortality decreased only modestly (Figure 525 ), likely because of a combination of screening, improved treatment, and better surveillance tools for those with prostate cancer. The increased gap between incidence and mortality largely represents overdiagnosis.26
Data from a multinational European RCT indicate that 1,055 men would have to be screened for nine years to prevent one death from prostate cancer (four prostate cancer deaths in the screened cohort vs. five in the unscreened cohort). Screening would result in the detection (and presumably treatment) of 37 additional men with prostate cancer.19 Of course, the “conundrum of overdiagnosis” is that at the time of diagnosis, clinicians cannot know with certainty who is overdiagnosed and who has clinically significant disease.26
Twenty-five years after the introduction of PSA testing, two lessons seem clear. First, a screening test for cancer should not be introduced into clinical practice until an RCT demonstrates that the test leads to reduced mortality; once a screening test is widely disseminated, it is virtually impossible to assemble a control group of persons who do not receive the test.18 Second, in the absence of conclusive evidence of net benefit or harm, PSA testing is likely to remain controversial unless and until it is replaced by a better biologic marker for early but aggressive prostate cancer.
LUNG CANCER
Lung cancer accounts for 27% of all cancer deaths in the United States; it will be diagnosed in 7% of Americans, and 6% will die from it. Among heavy smokers, lung cancer accounts for 33% of overall mortality.27 In the 1960s and 1970s, annual screening with chest radiography was commonly recommended in smokers because, as a result of lead-time and length-time bias, this seemed to improve survival. However, an RCT of annual radiography screening has since shown no reduction in lung cancer mortality.28
Beginning in 1999, published reports suggested that low-dose CT showed promise for the early detection of lung cancer. Unlike with PSA screening, these early reports did not lead to widespread screening but instead to rigorous testing in an RCT. The National Lung Screening Trial compared three rounds of annual low-dose CT with annual chest radiography in adults 55 to 74 years of age with at least a 30-pack-year smoking history. The trial found an RR of 0.80 for lung cancer mortality in the group screened with low-dose CT (the number needed to screen to prevent one lung cancer death over five years was 320), and for the first time in a cancer screening trial, there was an RR reduction in all-cause mortality (6.7%).29 Based largely on the results of this trial, the USPSTF recommends annual low-dose CT screening in persons 55 to 80 years of age with at least a 30-pack-year history who are otherwise healthy smokers or who have quit smoking within the previous 15 years.30
There are several caveats to low-dose CT screening. Only about 7 million of the 94 million current or previous smokers in the United States would have met the eligibility criteria for the National Lung Screening Trial. The trial showed that those in the lowest quintile of lung cancer risk experienced little or no benefit from screening31; therefore, smokers at lower risk than those in the trial are unlikely to have any benefit. More than 96% of all positive results in the National Lung Screening Trial were false positives. Although the radiation exposure from a single low-dose CT scan is comparable to that of a mammogram, the cumulative effect of annual screening for up to 25 years is unknown.27 Finally, low-dose CT leads to overdiagnosis of lung cancer,32 although the magnitude is likely less than with PSA screening. For all of these reasons, the AAFP concluded that the evidence is insufficient to endorse the USPSTF recommendation for low-dose CT screening.33
Perhaps the most important issue with low-dose CT screening is that it is a costly, high-tech response to what is essentially a behavioral and lifestyle problem. Smoking is responsible for 85% of lung cancers; convincing persons to quit smoking (or to not start) is far more effective in preventing lung cancer deaths than low-dose CT screening. Consequently, the USPSTF recommendation emphasizes that screening cannot prevent most lung cancer deaths, smoking cessation remains of primary importance, and low-dose CT screening should be performed only in the context of a screening program that includes smoking cessation interventions.30
Data Sources: The USPSTF (uspreventiveservicestaskforce.org), the primary source, was searched by specific cancer. Also searched were the Cochrane Library using the term cancer screening and Essential Evidence using the terms cancer screening and mass screening. Search dates: January to February 2014.