
Am Fam Physician. 2015;91(3):178-184
See related Practice Guidelines: Planning for Labor and Vaginal Birth After Cesarean Delivery: Guidelines from the AAFP.
Author disclosure: No relevant financial affiliations.
Nearly one-third of all deliveries in the United States are cesarean deliveries. Compared with spontaneous vaginal delivery, cesarean delivery is associated with increased maternal and neonatal morbidity and mortality. Interventions that decrease the chance of a cesarean delivery include avoiding non–medically indicated induction of labor, avoiding amniotomy, and having a doula present. In North America, the most common reasons for cesarean delivery include elective repeat cesarean delivery, dystocia or failure to progress, malpresentation, and fetal heart rate tracings that suggest fetal distress. Post–cesarean delivery complications include pain, endomyometritis, wound separation/infection, urinary tract infection, gastrointestinal problems, deep venous thrombosis, and septic thrombophlebitis. Women with no risk factors for deep venous thrombosis other than the postpartum state and the operative delivery do not require thromboembolism prophylaxis other than early ambulation. A pregnant woman's decision to attempt a trial of labor after cesarean delivery or have a planned repeat cesarean delivery involves a balancing of maternal and neonatal risks, as well as personal preference after counseling by her physician. Approximately 75% of attempted trials of labor after cesarean delivery are successful. Provision of advanced maternity care practices by family physicians, including serving as primary surgeons for cesarean deliveries, is consistent with the goals of the patient-centered medical home.
The cesarean delivery rate in the United States increased from 4.5% in 1965 to 32.9% in 2009.1,2 The increase is a result of both the higher rate of primary cesarean deliveries and the decrease in rates of vaginal birth after cesarean delivery (VBAC). Table 1 shows the range of cesarean delivery rates throughout the industrialized world.3
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Oral fluids should not be withheld after cesarean delivery. | A | 12 |
| Prophylactic antibiotics should be administered to all patients undergoing cesarean delivery. | A | 15, 16 |
| Women with no risk factors for deep venous thrombosis other than the postpartum state and the operative delivery should be mobilized early postoperatively. | B | 20 |
| For women with at least one additional risk factor for deep venous thrombosis, pharmacologic or mechanical prophylaxis (compression stockings or intermittent pneumatic compression device) should be used while the patient is in the hospital. | B | 20 |
| For women with multiple risk factors for deep venous thrombosis, pharmacologic prophylaxis should be combined with mechanical prophylaxis (intermittent pneumatic compression device). | B | 20 |
| Women at high risk of complications (e.g., those with a history of classical or T incision, uterine rupture, or extensive transfundal uterine surgery) and women in whom vaginal delivery is otherwise contraindicated (e.g., because of placenta previa) are not candidates for planned trial of labor after cesarean delivery. | C | 25 |
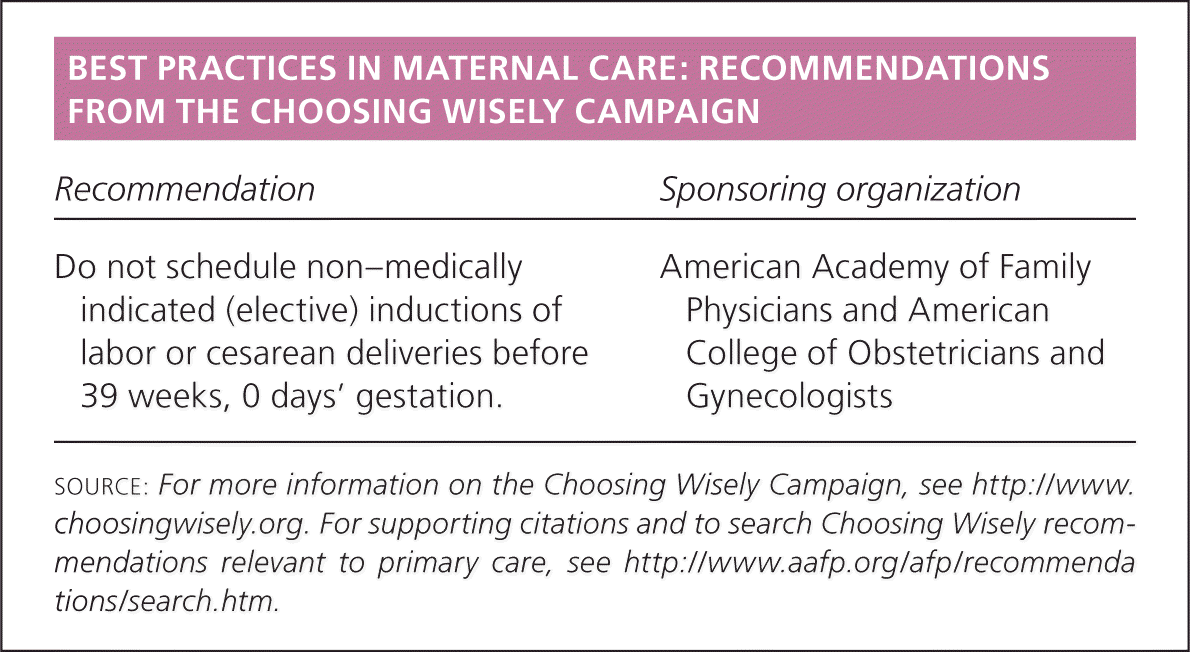
| Recommendation | Sponsoring organization |
|---|---|
| Do not schedule non–medically indicated (elective) inductions of labor or cesarean deliveries before 39 weeks, 0 days' gestation. | American Academy of Family Physicians and American College of Obstetricians and Gynecologists |
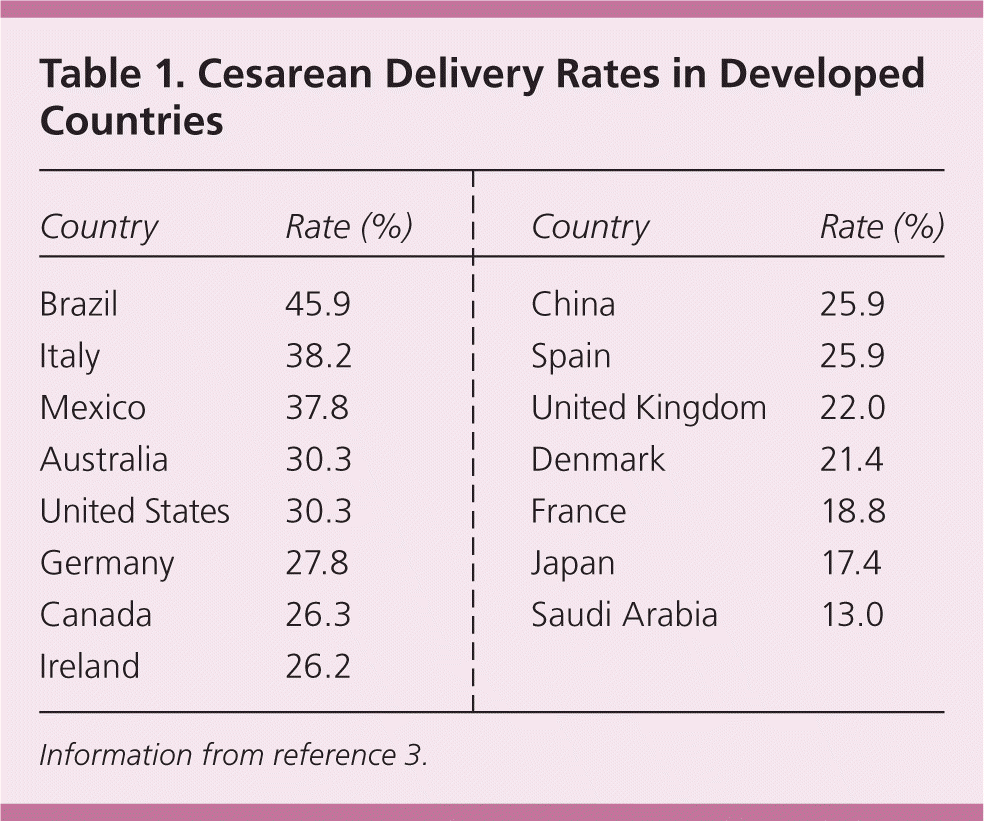
| Country | Rate (%) |
|---|---|
| Brazil | 45.9 |
| Italy | 38.2 |
| Mexico | 37.8 |
| Australia | 30.3 |
| United States | 30.3 |
| Germany | 27.8 |
| Canada | 26.3 |
| Ireland | 26.2 |
| China | 25.9 |
| Spain | 25.9 |
| United Kingdom | 22.0 |
| Denmark | 21.4 |
| France | 18.8 |
| Japan | 17.4 |
| Saudi Arabia | 13.0 |
The maternal mortality rate for a primary cesarean delivery is eight per 100,000 births4 and 13.4 per 100,000 births for elective repeat cesarean delivery (ERCD).5 One-half of these deaths are related to intraoperative complications. The most common causes of maternal death include complications from preeclampsia when it is the indication for cesarean delivery, pulmonary thromboembolism, amniotic fluid embolism, and hemorrhage.6
Prevention
Most nulliparous women have a strong preference for vaginal delivery.7 Pregnant women should be encouraged to take childbirth classes to prepare for the labor and delivery experience. Interventions that decrease the chance of a cesarean delivery include avoiding non–medically indicated induction of labor,8 avoiding amniotomy,9 and having an experienced doula present to provide continuous labor support and reassurance.10
Indications
Indications for cesarean delivery are listed in Table 2. The most common indications in North America are ERCD (30%), dystocia or failure to progress (30%), malpresentation (11%), and fetal heart rate tracings that suggest fetal distress (sinusoidal pattern or absent variability with recurrent late decelerations, recurrent variable decelerations, or bradycardia; 10%).11

| Abdominal cerclage |
| Active herpes outbreak |
| Congenital anomalies |
| Conjoined twins |
| Contracted pelvis (e.g., congenital or prior fracture) |
| Cord prolapse |
| Dystocia or failure to progress in labor (e.g., arrest of descent or dilation) |
| Elective repeat cesarean delivery |
| Fetal heart rate tracings that suggest fetal distress (sinusoidal pattern or absent variability with recurrent late decelerations, recurrent variable decelerations, or bradycardia) |
| Human immunodeficiency virus infection |
| Malpresentation (e.g., breech, brow, face/mentum posterior, transverse lie) |
| Medical conditions (e.g., cardiac, pulmonary, thrombocytopenia) |
| Obstructive pelvic tumor |
| Perimortem (mother in cardiac arrest) |
| Placenta previa |
| Placental abruption |
| Reconstructive vaginal surgery |
| Vasa previa |
Postoperative Care
Postoperative care after cesarean delivery (eTable A) is similar to that for any major abdominal surgery. The dressing should be removed after 24 hours and the wound monitored daily. Surgical clips can be removed and tape strips placed after three days for transverse skin incisions and after five to seven days for vertical incisions.

| Assess vital signs and fundal status every hour for four hours, every four hours for 24 hours, then every eight hours; massage the uterus per the same schedule and report extra lochia |
| Assess intake and output every four hours for 24 hours |
| Activity can occur ad libitum; encourage ambulation three times per day |
| The patient should cough and breathe deeply every hour when awake |
| Foley catheter to closed drainage; discontinue catheter the first postoperative morning or when the patient is ambulating well |
| Regular diet can resume as tolerated after nausea resolves |
| Administer dextrose 5% and lactated Ringer solution with 20 units of oxytocin (Pitocin), 1 L given at 125 mL per hour for two bags, then dextrose 5% and lactated Ringer solution at 125 mL per hour |
| Convert to heparin lock when the patient is tolerating oral intake well |
| Administer intravenous pain medication, such as morphine 2 to 8 mg intravenously every two hours as needed |
| Administer antinausea/antiemetic therapy, such as promethazine 25 to 50 mg intramuscularly every four hours as needed |
| Administer oral pain medication, such as oxycodone/acetaminophen (Percocet) one to two tablets every three to four hours as needed, after tolerating oral intake |
| Laboratory tests: first postoperative morning, measure hemoglobin and hematocrit levels |
| Administer RhO(D) immune globulin (Rhogam) if indicated by infant cord blood Rh status |
| Administer rubella, hepatitis, and Tdap (tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis) vaccines at discharge if indicated |
Pain management is an important aspect in early postoperative care. Initial pain control may be obtained with narcotics provided preoperatively via intrathecal administration or postoperatively by patient-controlled anesthesia pumps. Patients should be transitioned to oral narcotics and nonsteroidal anti-inflammatory drugs once they are able to eat and drink. Incisional pain, which often worsens with activity, can be controlled with scheduled use of oral medications.
Although it has been customary for fluids or food to be withheld for a period of time after abdominal operations, this policy has no advantages over early feeding after uncomplicated cesarean delivery.12 Fertility planning should be discussed before discharge and again at the six-week follow-up visit.
RETURN TO ACTIVITY
Recommendations for activity after cesarean delivery are based on tradition and anecdote. The available data do not support many of the recommendations currently provided.13 Patients should receive consistent pre- and postprocedure counseling, and an adequate postoperative analgesic regimen.
There is no retrospective or prospective evidence regarding driving after cesarean delivery. Cognitive function and use of narcotic analgesics, rather than wound-related concerns, should determine if and when patients can drive. Women may resume driving when they are comfortable with the hand and foot movements required for driving.13
There is limited retrospective and prospective evidence regarding exercise after cesarean delivery. Forceful coughing increases intra-abdominal pressure as much as jumping jacks.14 Patients can resume preprocedure exercise levels when they feel comfortable doing so, although the programs should be tailored for postpartum women.13
There is no consistent retrospective evidence and no prospective evidence regarding vaginal intercourse after cesarean delivery. Women and their partners should make the decision to resume intercourse mutually, and should use vaginal lubricants and sexual positions that permit the woman to control the depth of vaginal penetration.13
There is no consistent prospective or retrospective evidence regarding return to work. Women should weigh personal comfort, bonding with their newborn, and familial considerations when making the decision. A graded return should be considered.13
Early Postoperative Complications
Infection is the most common complication within the first 10 days after cesarean delivery. The rate of infection without prophylactic antibiotics approaches 85%, whereas the infection rate with prophylactic antibiotics is only about 5%.15 Prophylactic antibiotics should be administered to all patients undergoing cesarean delivery; a single dose of a first-generation cephalosporin or ampicillin is effective.15,16
ENDOMYOMETRITIS
Endomyometritis is a clinical diagnosis that presents as uterine tenderness, fever (two postoperative temperatures higher than 100°F [38°C] more than 24 hours after delivery), and leukocytosis. Overall, 90% to 95% of cases will resolve within 72 hours with a combination of broad-spectrum intravenous antibiotics, such as clindamycin and an aminoglycoside, or with a third-generation cephalosporin alone.17 A small percentage of patients will develop further complications.
WOUND SEPARATION/INFECTION
Wound separation after cesarean delivery occurs in approximately 5% of cases. It may involve the skin through fascia. Nearly two-thirds of the wounds that open are infected.18
Wound infection presents as erythema and tenderness, and may develop purulence and cause fever. Wound infection is a clinical diagnosis with laboratory results serving as an adjunct. Treatment includes broad-spectrum antibiotics. The wound may need to be probed, opened, irrigated, and packed, and necrotic tissue debrided.
Fascial dehiscence is uncommon (0.3% of cesarean deliveries), but it occurs in approximately 6% of open wounds.18 It presents as copious discharge and may be followed by protrusion of the bowel. If this occurs, the bowel should be covered with moist sterile gauze pad, and surgical consultation should be obtained immediately.
URINARY TRACT INFECTION
Urinary tract infections are often associated with use of an indwelling catheter. Treatment should be initiated with broad-spectrum antibiotics and subsequent antibiotic therapy based on urine culture sensitivity results.
GASTROINTESTINAL COMPLICATIONS
An ileus presents as abdominal distention, nausea, vomiting, and failure to pass flatus. Bowel sounds may be absent. Radiographic studies show distended loops of small and large bowel, with gas usually present in the colon. Treatment involves withholding oral intake, awaiting the return of bowel function, and providing adequate fluids and electrolytes.
In contrast, obstruction presents as high-pitched bowel sounds and peristaltic rushes. Radiographic studies show single or multiple loops of distended bowel, usually in the small bowel, with air-fluid levels. The patient may need nasogastric suctioning or a duodenal-jejunal tube. Surgical consultation is warranted if an obstruction persists despite conservative management.
THROMBOEMBOLIC COMPLICATIONS
Deep venous thrombosis (DVT) is three to five times more common after cesarean delivery than after vaginal delivery.19 DVT can progress to pulmonary embolus if untreated. It typically presents as unilateral leg tenderness, swelling, and a palpable cord.
The American College of Chest Physicians recommends early ambulation in women with no risk factors for DVT other than the postpartum state and the operative delivery.20 For women with at least one additional risk factor, pharmacologic prophylaxis with low-molecular-weight heparin or mechanical prophylaxis (compression stockings or intermittent pneumatic compression device) while the patient is in the hospital is recommended.20 For women with multiple risk factors, pharmacologic and mechanical prophylaxis should be initiated.20 In patients receiving pharmacologic prophylaxis before delivery, it should be discontinued at least 24 hours before scheduled cesarean delivery and resumed six to 12 hours after delivery.20,21 Table 3outlines a risk stratification approach to thromboembolism prophylaxis.22

| Low risk: early ambulation |
| Cesarean delivery for uncomplicated pregnancy with no other risk factors |
| Moderate risk: low-molecular-weight heparin or compression stockings |
| Age > 35 yr |
| Obesity (BMI > 30)* |
| Parity > 3 |
| Gross varicose veins |
| Current infection |
| Preeclampsia |
| Immobility for > 4 days before operation |
| Major current illness |
| Emergency cesarean delivery during labor |
| High risk: low-molecular-weight heparin and compression stockings |
| Presence of more than two risk factors from the moderate-risk section |
| Cesarean hysterectomy |
| Previous deep vein thrombosis or known thrombophilia |
SEPTIC THROMBOPHLEBITIS
Septic thrombophlebitis is a diagnosis of exclusion. Persistent and unexplained fever is often the only symptom, although some patients have pelvic pain. Findings on physical examination, ultrasonography, and computed tomography are often negative. Continued fever despite several days of antibiotic therapy suggests septic thrombophlebitis. Traditional treatment includes intravenous heparin in combination with antibiotics; however, evidence of benefit is lacking.23
Trial of Labor After Cesarean Delivery
The decision by a pregnant woman to attempt a trial of labor after cesarean delivery (TOLAC) or a planned ERCD involves balancing individual maternal and neonatal risks, as well as personal preference after counseling by her physician. A thorough understanding of available evidence is essential in providing this counseling to patients.
The American Academy of Family Physicians (AAFP) recently published a clinical practice guideline that provides an evidence-based approach to counseling women about labor after a previous cesarean delivery.24 The multidisciplinary panel recommends that patients deciding between TOLAC and ERCD receive an individualized assessment and subsequent counseling about the benefits and risks of each. The panel also concluded that a planned trial of labor is an option for most women with a previous cesarean delivery.
The full guideline can be accessed at https://www.aafp.org/pvbac. In addition, a summary appearing in the Annals of Family Medicine can be accessed at http://www.annfammed.org/content/13/1/80.
Documentation of counseling and the management plan should be included in the medical record. During the informed consent process, at least three basic issues need to be addressed. These are the patient's plan for future family size, the chance of successful VBAC, and safety concerns.
Women at high risk of complications (e.g., those with a history of classical or T incision, uterine rupture, or extensive transfundal uterine surgery) and women in whom vaginal delivery is otherwise contraindicated (e.g., because of placenta previa) are not candidates for planned TOLAC.25
PLANNED FAMILY SIZE
There is no difference in risk of peripartum hysterectomy between planned cesarean delivery and planned vaginal delivery. However, there is a significantly increased risk of placenta previa, placenta accreta, and placenta previa with accreta after a woman's first cesarean, and a significantly increased risk of the need for gravid hysterectomy after a woman's second cesarean delivery.26 Additionally, death rates increase from eight in 100,000 deliveries with the first cesarean delivery to 39 in 100,000 with the fourth cesarean delivery.4
CHANCE FOR SUCCESS
Approximately 75% of attempted TOLACs will be successful, although this rate varies depending on the clinical situation that led to the first cesarean delivery.5 The likelihood of VBAC is highest in women with a previous vaginal delivery, a body mass index of 25 kg per m2 or less, previous cesarean delivery for breech presentation, spontaneous onset of labor with ripe cervix, and age younger than 35 years (Table 4).7

| Decreased success (< 60%)* |
| Two or more prior cesarean sections without a vaginal delivery |
| Cesarean section for failure to descend in second stage |
| Labor induction required |
| Infant 4,000 g or more |
| Body mass index greater than 40 kg/m2 |
| Maternal age older than 35 years |
| Neutral success (65%–75%) |
| Gestational age older than 40 weeks |
| Prior cesarean delivery for nonreassuring fetal monitoring |
| Unknown scar |
| Twins (limited data) |
| Labor augmentation |
| Two prior cesarean sections with history of vaginal birth (limited data) |
| Cesarean section for failure to progress in first stage of labor |
| Body mass index 25–40 kg/m2 |
| Increased success (>75%) |
| Prior successful vaginal birth |
| BMI 25 kg/m2 or less |
| Prior cesarean delivery for breech presentation |
| Spontaneous labor with ripe cervix by Bishop score |
| Maternal age younger than 35 years |
SAFETY
The benefits of VBAC is decreased maternal risk associated with vaginal delivery (e.g., decreased blood loss and lower risk of transfusion, decreased risk of DVT, decreased risk of infection) and a quicker recovery period, with decreased length of hospitalization.
Planned ERCD and planned VBAC are both associated with benefits and harms. Evidence for the risks and benefits of TOLAC vs. ERCD are predominantly from retrospective cohort studies.27 The rate of perinatal mortality associated with TOLAC is similar to that in infants born to nulliparous women in labor (1.3 per 1,000 births with TOLAC compared with 0.5 per 1,000 births with ERCD).28
UTERINE DEHISCENCE OR RUPTURE
Uterine dehiscence, asymptomatic scar separation that does not penetrate the serosa or produce hemorrhage, occurs in 12.6 per 1,000 TOLACs. This rate is comparable to that in women undergoing ERCD.28 Uterine rupture is a through-and-through scar separation that is clinically symptomatic and requires surgical intervention. The overall rate of uterine rupture during TOLAC is 0.7%.29,30 eTable B lists the factors that influence the risk of uterine rupture during TOLAC.

| Decreased (< 1%)* |
| Prior vaginal delivery |
| Low uterine segment incision from prior cesarean |
| Preterm delivery |
| Two-layer closure of uterine incision |
| Neutral (1%–2%) |
| Induction of labor with good Bishop score with oxytocin |
| One-layer uterine closure |
| Gestational age of more than 40 weeks |
| Low vertical uterine incision (limited data; could be increased to up to 5%) |
| Unknown uterine scar without high risk for prior classical incision |
| Increased (> 2%–4%) |
| Unknown scar in the setting of high risk for prior classical incision (e.g., preterm abnormal lie or term transverse lie) |
| Classical or T uterine incision (4%–9%) |
| Lower uterine segment thickness less than 1.5 mm on ultrasound at term |
| Prior myomectomy, cornual resection, or other full-thickness uterine surgery |
| Prior uterine rupture |
| Morbid obesity (body mass index ≥ 40 kg/m2) |
| Two or more prior uterine incisions without vaginal delivery |
| Induction of labor with poor Bishop score with prostaglandin agent or oxytocin |
Fetal bradycardia is the most common and characteristic clinical manifestation of uterine rupture.31 Variable or late decelerations may precede the bradycardia, but there is no fetal heart rate pattern that is pathognomonic of rupture.
Maternal manifestations, including vaginal bleeding, are variable. In women with known uterine scarring or trauma, uterine rupture should be suspected with constant abdominal pain and signs of intra-abdominal hemorrhage. Other clinical manifestations include signs of shock, cessation of uterine contractions, loss of station of the fetal presenting part, uterine tenderness, and change in uterine shape.
Treatment of uterine rupture is largely dependent on the patient's hemodynamic status and desire for future fertility. In some cases, a layered closure of the myometrium is sufficient, although hysterectomy may be necessary.
FACILITY LIMITATIONS
The American College of Obstetricians and Gynecologists (ACOG) recommends that TOLAC be undertaken at facilities capable of emergency surgical deliveries.25 The Northern New England Perinatal Quality Improvement Network VBAC guidelines use a three-tiered, risk-based system that can be modified for capabilities of individual facilities and availability of resources, and as new data emerge (Table 5).32
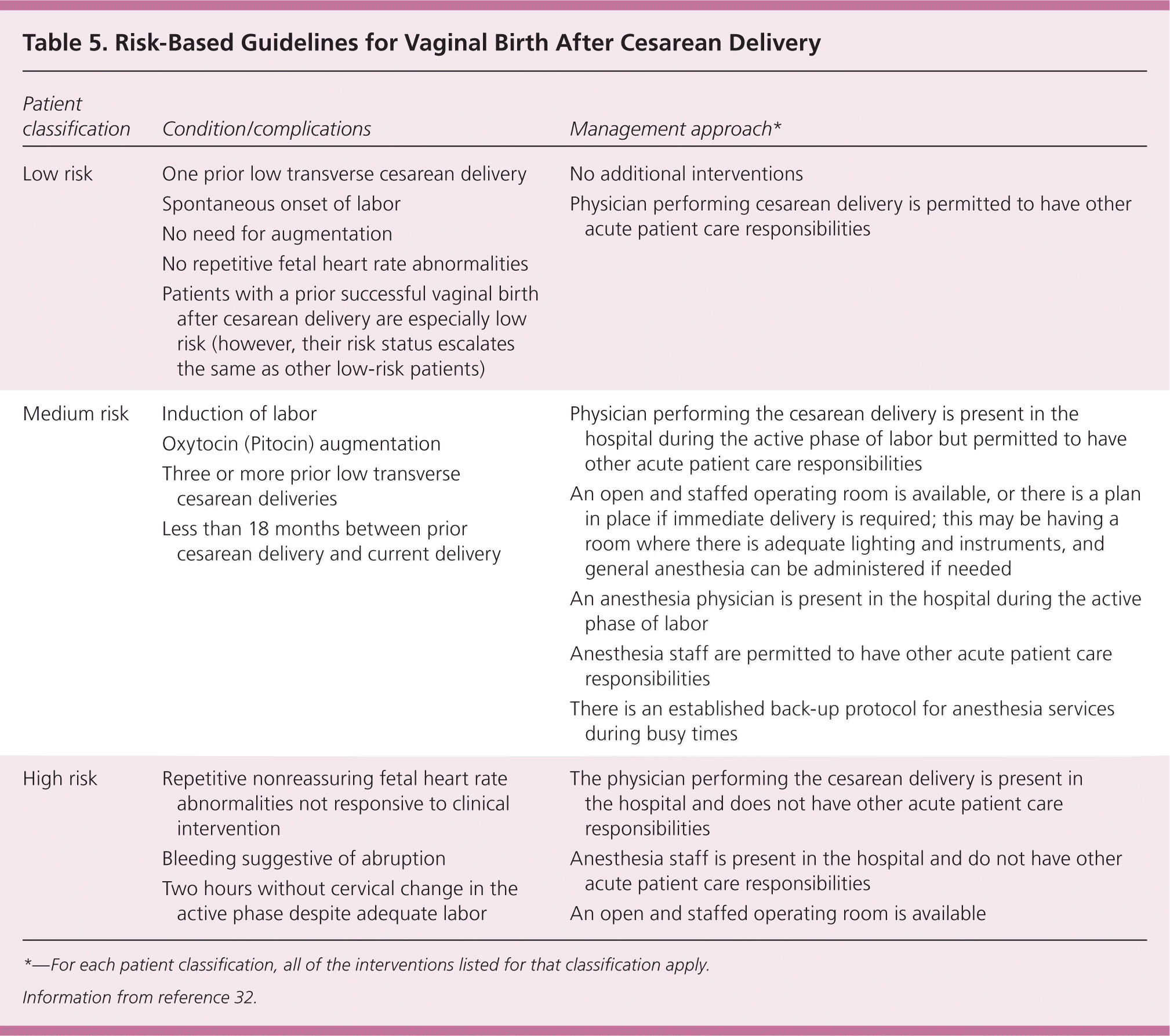
| Patient classification | Condition/complications | Management approach* |
|---|---|---|
|
|
|
|
|
|
|
|
|
The AAFP's guideline development panel specifically reviewed available data related to resources required at a hospital to offer TOLAC. They found that the limited data available demonstrate similar outcomes for women with TOLAC irrespective of hospital delivery volume, location, or type. The panel recommended that all women be counseled about the capabilities of the facility in which they plan to deliver, and that high risk women be referred to a hospital able to provide emergent care as required.24
Family Physicians as Primary Surgeons
The joint AAFP and ACOG recommended curriculum guidelines for family medicine residents describe core and advanced obstetric training for family physicians.33 Throughout the United States, more than 2,000 family physicians perform cesarean deliveries as the primary surgeon.34 Family physicians are the only surgeons in some rural areas, and provision of advanced maternity care practices by family physicians is consistent with the goals of the patient-centered medical home. There are more than 30 fellowships for family physicians wanting training to perform cesarean deliveries as the primary surgeon. Published studies indicate that family physicians performing cesarean deliveries meet or exceed national standards.35 The AAFP's position paper on cesarean delivery in family medicine can be accessed at https://www.aafp.org/about/policies/all/cesarean-delivery.html.
Data Sources: In addition to a literature search completed in 2011 for the American Academy of Family Physicians' Advanced Life Support in Obstetrics Chapter Q: Cesarean Delivery published in March 2012, we searched the Cochrane Database of Systematic Reviews, the National Guideline Clearinghouse, and PubMed using the key words cesarean delivery, vaginal birth after cesarean, trial of labor after cesarean delivery, family physician, and family medicine residency, individually and in combination. Search dates: January 27, 2012, and July 25, 2014.
This article is one in a series on “Advanced Life Support in Obstetrics (ALSO),” coordinated by Larry Leeman, MD, MPH, ALSO Managing Editor, Albuquerque, N.M.
