
A more recent article on pressure injuries is available.
This is an updated version of the article that appeared in print.
Am Fam Physician. 2015;92(10):888-894
Author disclosure: No relevant financial affiliations.
Patients with limited mobility due to physical or cognitive impairment are at risk of pressure ulcers. Primary care physicians should examine at-risk patients because pressure ulcers are often missed in inpatient, outpatient, and long-term care settings. High-risk patients should use advanced static support surfaces to prevent pressure ulcers and air-fluidized beds to treat pressure ulcers. Physicians should document the size and clinical features of ulcers. Cleansing should be done with saline or tap water, while avoiding caustic agents, such as hydrogen peroxide. Dressings should promote a moist, but not wet, wound healing environment. The presence of infection is determined through clinical judgment; if uncertain, a tissue biopsy should be performed. New or worsening pain may indicate infection of a pressure ulcer. When treating patients with pressure ulcers, it is important to keep in mind the patient's psychological, behavioral, and cognitive status. The patient's social, financial, and caregiver resources, as well as goals and long-term prognosis, should also be considered in the treatment plan.
Pressure ulcers are injuries of the skin or underlying tissue that occur as a result of pressure alone or in combination with shearing forces. The ulcers typically occur over boney prominences.1 During pressure ulcer development, external pressure exceeds capillary blood flow pressure, leading to ischemia and tissue injury.2 Pressure ulcers are common, affecting up to 3 million Americans3 and costing about $11 billion annually in the United States.4 The most important risks for pressure ulcer development are impaired mobility, decreased perfusion, and edema or a stage I pressure ulcer.5 Additional risk factors include poor general health status, low nutritional status, and skin moisture from conditions such as incontinence.5 Advanced age and cognitive impairment are also risk factors.5 Multifactorial interventions to reduce pressure ulcers are among the top-10 strategies strongly encouraged by the Agency for Healthcare Research and Quality for adoption in all health care systems.6 When treating patients with pressure ulcers, it is important to keep in mind the patient's psychological, behavioral, and cognitive status. The patient's social, financial, and caregiver resources, as well as goals and long-term prognosis, should also be considered when developing a treatment plan.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Multicomponent interventions should be used to prevent pressure ulcers in high-risk patients. | B | 7 |
| Advanced static support surfaces, rather than standardized hospital mattresses, should be used to prevent pressure ulcers in high-risk patients. | A | 3, 8, 9 |
| Hydrocolloid or foam dressings should be used for the treatment of pressure ulcers. | B | 4, 22 |
| Cleansing pressure ulcers with caustic agents, such as povidone-iodine (Betadine) or Dakin's solutions, should be avoided. | C | 23, 24 |
| Air-fluidized beds should be used for the treatment of pressure ulcers. | A | 4 |
| New or worsening pain may indicate infection of a pressure ulcer. | C | 32 |
What Are the Most Effective Ways to Prevent Pressure Ulcers in Hospitalized or Immobile Patients?
EVIDENCE SUMMARY
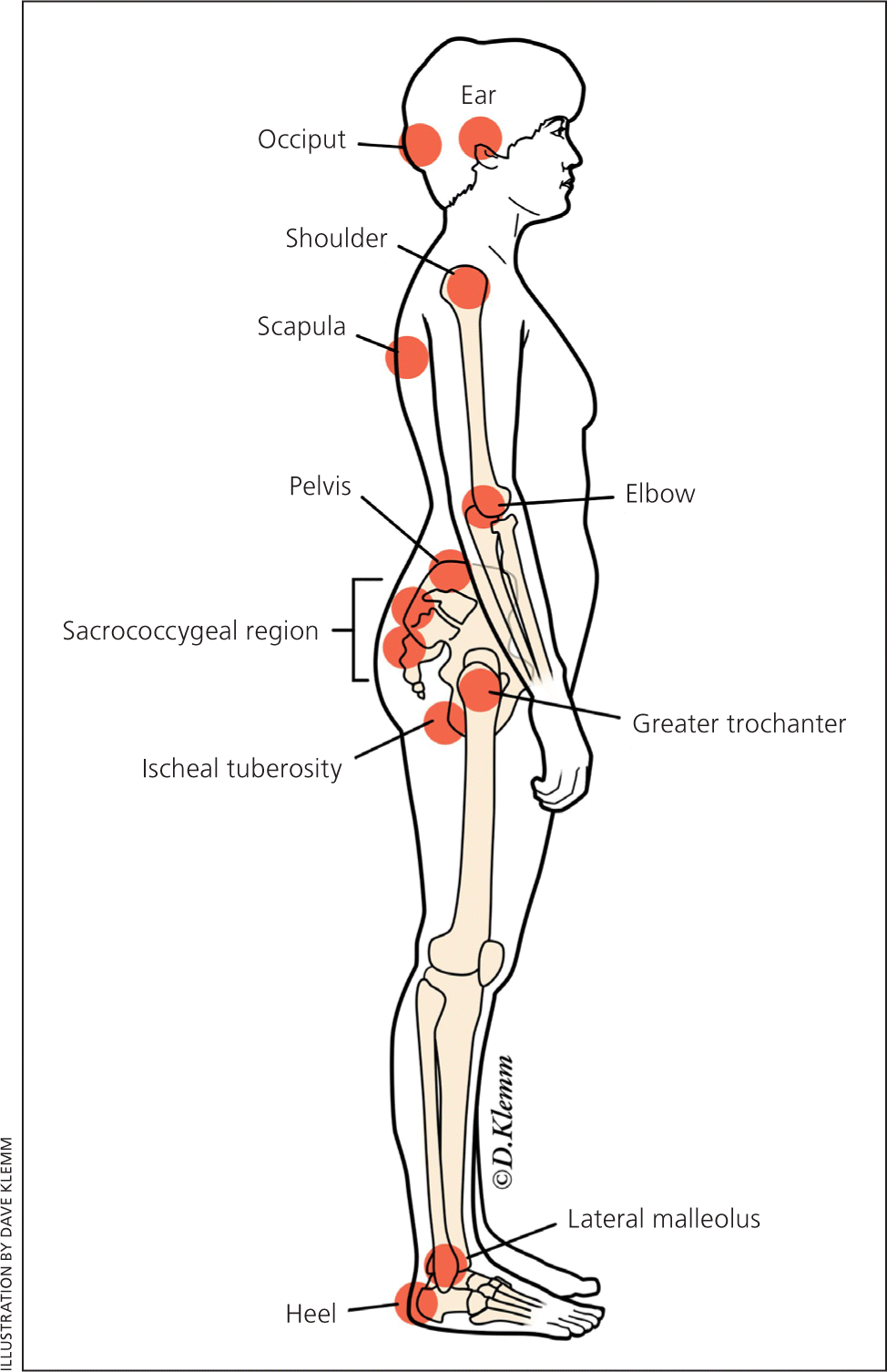
Pressure ulcers may develop in as little as four to six hours.10 Primary care physicians should examine at-risk patients periodically because ulcers are often missed in inpatient, outpatient, and long-term care settings. The most common sites of pressure ulcers are indicated in Figure 1. It is important to identify patients at high risk, initiate preventive measures, and monitor for ulcer development. Moderate-quality evidence from a systematic review of 26 studies supports a multicomponent intervention to prevent pressure ulcers. This usually includes an assessment, use of a specialized support surface, repositioning, mobilization, avoiding friction, and management of nutrition and moisture. Other features include an on-site clinician to lead the program, a multidisciplinary team, audit and feedback, ongoing education, simplification, and standardization.7 Multidisciplinary care is crucial, including primary care physicians, nurses (including those specializing in wound care), social workers, podiatrists, surgeons, dietitians, physical and occupational therapists, and infectious disease consultants as needed.
The Braden, Waterlow, and Norton scales are commonly used for pressure ulcer risk assessment. These are five- to six-item scales that assess risk factors such as physical and mental condition, mobility, moisture, continence, and nutrition. However, they are weak predictors of the risk of ulcers, and may be no better at reducing the risk of formation than a nurse's or physician's clinical judgment.3,9,11 The 2015 clinical practice guideline from the American College of Physicians suggests that risk scales may be most useful to clinicians with limited experience in caring for patients who develop pressure ulcers.9
Adequate evidence supports the use of advanced static support surfaces rather than standard hospital mattresses to prevent pressure ulcers in high-risk patients.3,8,9 These support surfaces include foam, water, gel, and air mattresses or mattress overlays. Other commonly used measures include repositioning, nutritional supplementation, creams, dressings, and pads placed over boney prominences. There is inadequate evidence on the effectiveness of these measures, although all have a low risk of harm.3,9,12,13 There is also no evidence to suggest an optimal interval at which to reposition patients,14,15 although every two hours is recommended based on expert opinion.16 It is reasonable to consider consultation with a dietitian and use of skin moisturizers to prevent pressure ulcers.17 Even with optimal care, pressure ulcers may still develop in certain patients.
How Should Pressure Ulcers Be Assessed?
Clinicians should take a medical and pressure-ulcer history, including assessment of pain. They should examine and measure the length, width, and depth of ulcers. The presence of exudate, necrotic tissue, eschar, slough, undermining, and tunneling or sinus tracts should be documented. The presence of healing tissue (pink granulation tissue or epithelialization) should be noted. The wound should be staged using the National Pressure Ulcer Advisory Panel's 1 to 4 staging system.18 [updated]
EVIDENCE SUMMARY
The most commonly used staging system for pressure ulcers is the National Pressure Ulcer Advisory Panel staging system (Table 1).18,19 This system was recently updated, and now uses Arabic numerals and refers to "pressure injury" rather than "pressure ulcer", since not all injuries involve ulceration of the tissue. There are four stages of pressure ulcers. In a stage 1 injury, the skin is intact with non-blanchable redness, or differing color from surrounding skin in darkly pigmented skin. The area may be warm, cool, soft, firm, or painful compared with other areas. Patients with stage 1 injuries are at risk of developing additional pressure injuries. Stage 2 pressure injuries involve partial-thickness skin loss with exposed dermis. They are shallow and have a red-pink wound bed. An intact blister is also considered a stage 2 injury. There should be no slough (dead tissue that is often a yellow-gray color and tightly adhered) or bruising in a stage 2 ulcer.
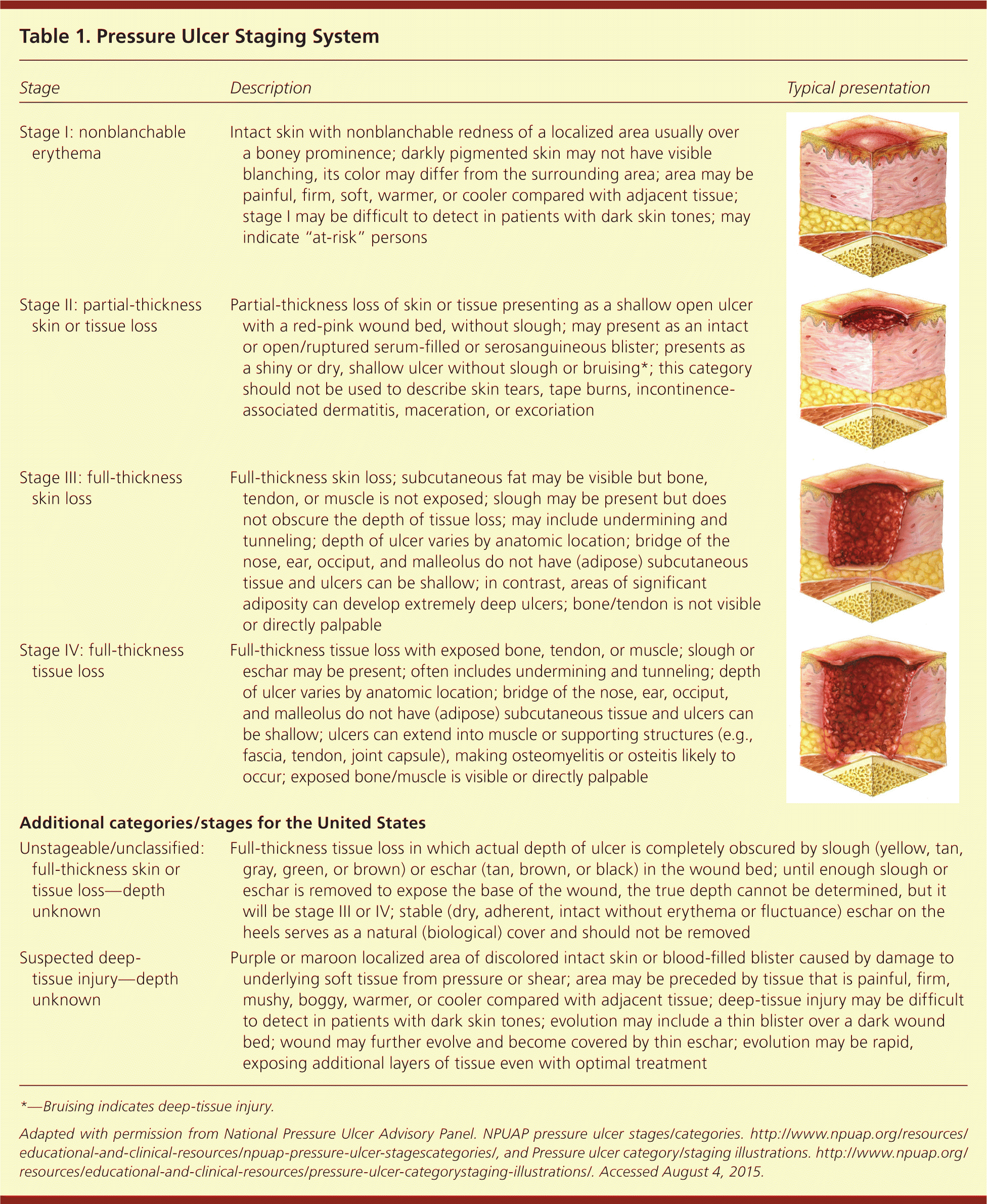
| Stage | Description | Typical presentation |
|---|---|---|
| Stage 1: nonblanchable erythema of intact skin | Intact skin with nonblanchable redness of a localized area usually over a boney prominence; darkly pigmented skin may not have visible blanching, its color may differ from the surrounding area; area may be painful, firm, soft, warmer, or cooler compared with adjacent tissue; stage I may be difficult to detect in patients with dark skin tones; may indicate “at-risk” persons | |
| Stage 2: partial-thickness skin loss with exposed dermis | Partial-thickness loss of skin or tissue presenting as a shallow open ulcer with a red-pink wound bed, without slough; may present as an intact or open/ruptured serum-filled or serosanguineous blister; presents as a shiny or dry, shallow ulcer without slough or bruising*; this category should not be used to describe skin tears, tape burns, incontinence-associated dermatitis, maceration, or excoriation | |
| Stage 3: full-thickness skin loss | Full-thickness skin loss; subcutaneous fat may be visible but bone, tendon, or muscle is not exposed; slough may be present but does not obscure the depth of tissue loss; may include undermining and tunneling; depth of ulcer varies by anatomic location; bridge of the nose, ear, occiput, and malleolus do not have (adipose) subcutaneous tissue and ulcers can be shallow; in contrast, areas of significant adiposity can develop extremely deep ulcers; bone/tendon is not visible or directly palpable | |
| Stage 4: full-thickness skin and tissue loss | Full-thickness tissue loss with exposed bone, tendon, or muscle; slough or eschar may be present; often includes undermining and tunneling; depth of ulcer varies by anatomic location; bridge of the nose, ear, occiput, and malleolus do not have (adipose) subcutaneous tissue and ulcers can be shallow; ulcers can extend into muscle or supporting structures (e.g., fascia, tendon, joint capsule), making osteomyelitis or osteitis likely to occur; exposed bone/muscle is visible or directly palpable | |
| Additional categories/stages for the United States | ||
| Unstageable pressure injury: obscured full-thickness skin and tissue loss | Full-thickness tissue loss in which actual depth of ulcer is completely obscured by slough (yellow, tan, gray, green, or brown) or eschar (tan, brown, or black) in the wound bed; until enough slough or eschar is removed to expose the base of the wound, the true depth cannot be determined, but it will be stage 3 or 4; stable (dry, adherent, intact without erythema or fluctuance) eschar on the heels serves as a natural (biological) cover and should not be removed | |
| Deep-tissue pressure injury: persistent non-blanchable deep red, maroon or purple discoloration | Purple or maroon localized area of discolored intact skin or blood-filled blister caused by damage to underlying soft tissue from pressure or shear; area may be preceded by tissue that is painful, firm, mushy, boggy, warmer, or cooler compared with adjacent tissue; deep-tissue injury may be difficult to detect in patients with dark skin tones; evolution may include a thin blister over a dark wound bed; wound may further evolve and become covered by thin eschar; evolution may be rapid, exposing additional layers of tissue even with optimal treatment | |
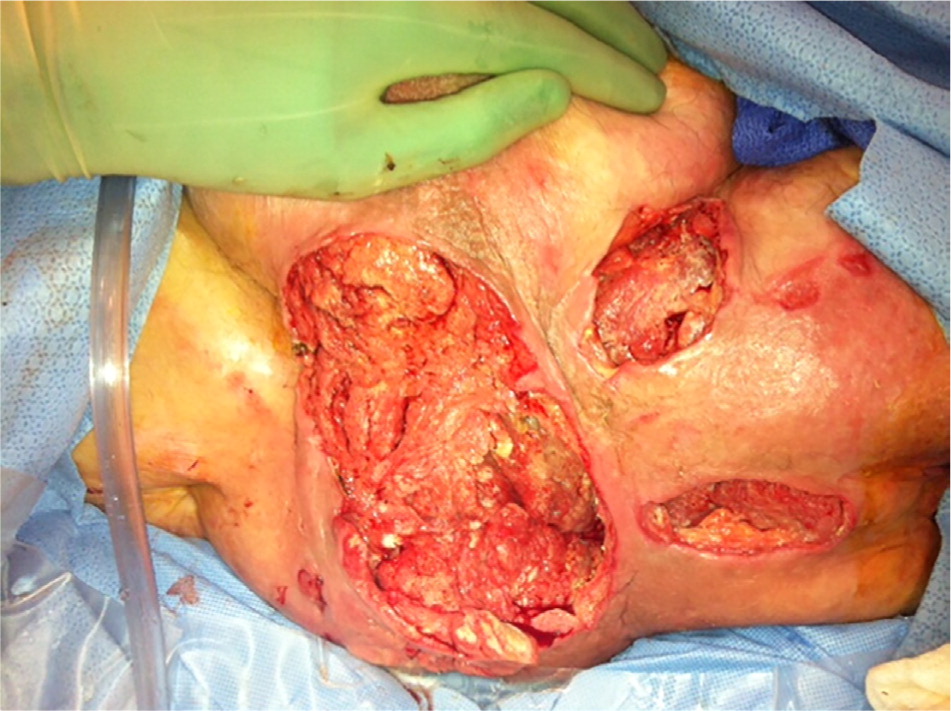
A stage 3 injury involves full-thickness skin loss. It may be shallow or deep, and may have undermining or tunneling. A stage 4 injury involves full-thickness skin and tissue loss of the dermis with exposed bone, tendon, or muscle (Figure 2). Additionally, injuries may be classified as “unstageable: obscured full-thickness skin and tissue loss” if slough or eschar is present, because true depth and staging cannot be determined until it is removed. Injuries may also be classified as “deep-tissue pressure injury.”18
What Is the Best Way to Cleanse and Dress a Pressure Ulcer?
Pressure ulcers should be cleansed with saline or tap water.1,20,21 They should be debrided of slough and necrotic tissue. A hydrocolloid, foam, or other nonadherent dressing that promotes a moist wound environment should be used.4,22 Wet-to-dry dressings, povidone-iodine (Betadine) solution, and Dakin's solution should be avoided.23,24 Pain associated with cleansing or dressing pressure ulcers should be treated appropriately.
EVIDENCE SUMMARY
There is insufficient evidence to advise using one cleansing solution or technique over another.20 In general, saline is favored to cleanse pressure ulcers, but tap water may be used if saline is not available.1,21 Solutions that may impede granulation tissue formation, such as hydrogen peroxide, chlorine-based solutions (Dakin's), or povidone-iodine solution, should be avoided.23,24 However, a time-limited course of properly diluted antiseptics may be used in an infected wound to control bacterial burden or in palliative situations with wounds that are not expected to heal and have a foul odor caused by a heavy bacterial burden.1
Debridement of necrotic tissue and slough is important to promote healing and decrease the risk of infection. Debridement can be autolytic (provided by the wound's own drainage), enzymatic (collagenase is commonly available and well tolerated), mechanical (whirlpool), or surgical (sharp debridement).1
A moist wound environment is believed to assist in healing and aid in autolytic debridement.25 Low-quality evidence supports the use of a hydrocolloid or foam dressing over standard therapy.4,22 Hydrocolloid dressings are most useful with light to moderate wound drainage, and foam dressings are available in a range of thicknesses for drier wounds to those with heavier exudate.26 If these are not available, it is important that any dressing promote moist (but not wet) wound healing and be nonadhesive. Wet-to-dry dressings should be avoided because the debridement from removal of a fully dry dressing may impede healing and can cause significant pain.1
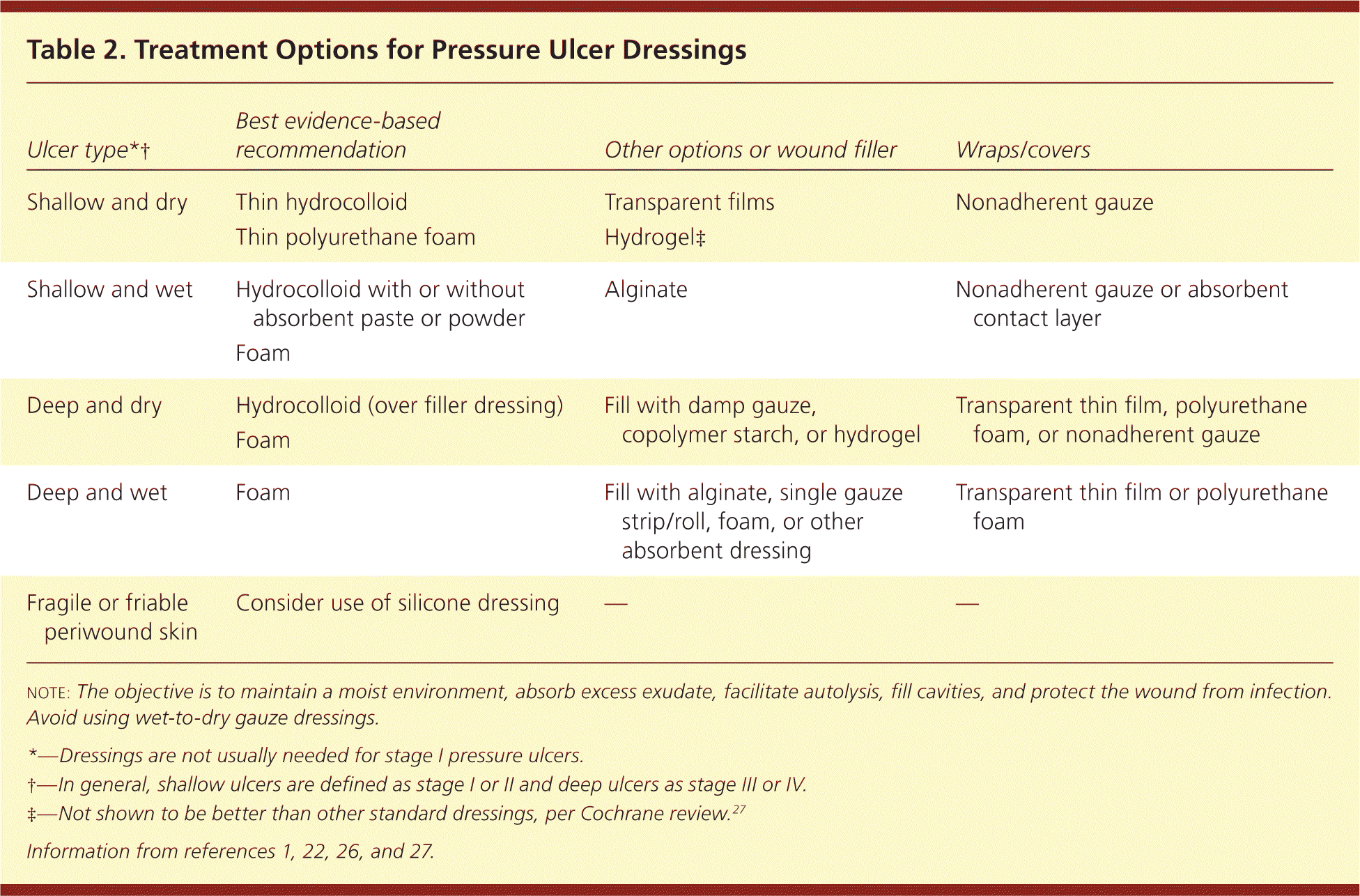
| Ulcer type*† | Best evidence-based recommendation | Other options or wound filler | Wraps/covers |
|---|---|---|---|
| Shallow and dry | Thin hydrocolloid | Transparent films | Nonadherent gauze |
| Thin polyurethane foam | Hydrogel‡ | ||
| Shallow and wet | Hydrocolloid with or without absorbent paste or powder | Alginate | Nonadherent gauze or absorbent contact layer |
| Foam | |||
| Deep and dry | Hydrocolloid (over filler dressing) | Fill with damp gauze, copolymer starch, or hydrogel | Transparent thin film, polyurethane foam, or nonadherent gauze |
| Foam | |||
| Deep and wet | Foam | Fill with alginate, single gauze strip/roll, foam, or other absorbent dressing | Transparent thin film or polyurethane foam |
| Fragile or friable periwound skin | Consider use of silicone dressing | — | — |
Does Nutritional Supplementation Improve Prognosis?
EVIDENCE SUMMARY
A 2013 systematic review and a 2015 guideline by the American College of Physicians cite moderate-quality evidence supporting protein supplementation to treat pressure ulcers.4,22 However, a 2014 Cochrane review concluded that there is no evidence that nutritional interventions, including protein, provide benefit in the prevention or treatment of pressure ulcers.28 Although commonly prescribed, only one good-quality study has evaluated the effect of vitamin C supplementation, and it revealed no change in the rate of wound healing. Insufficient evidence was found on the effect of zinc supplementation.4,22
Which Other Interventions May Help When Treating Pressure Ulcers?
Relieving the source of pressure, repositioning the patient, and using air-fluidized beds are recommended to treat pressure ulcers.4,22 Moderate-strength evidence supports the use of radiant heat dressings and electrical stimulation.4,22 Alternating-pressure surfaces, platelet-derived growth factor, and light therapy may also help.4,22 Surgical consultation should be considered for advanced ulcers not responding to standard therapy.
EVIDENCE SUMMARY
Treatment of pressure ulcers should alleviate the causes and risks contributing to ulcer formation. Clinicians should attempt to remove all pressure over the ulcer. This may include simple techniques such as “floating heels” (Figure 3) and frequent repositioning (every two hours). However, repositioning has not been studied in randomized controlled trials.29
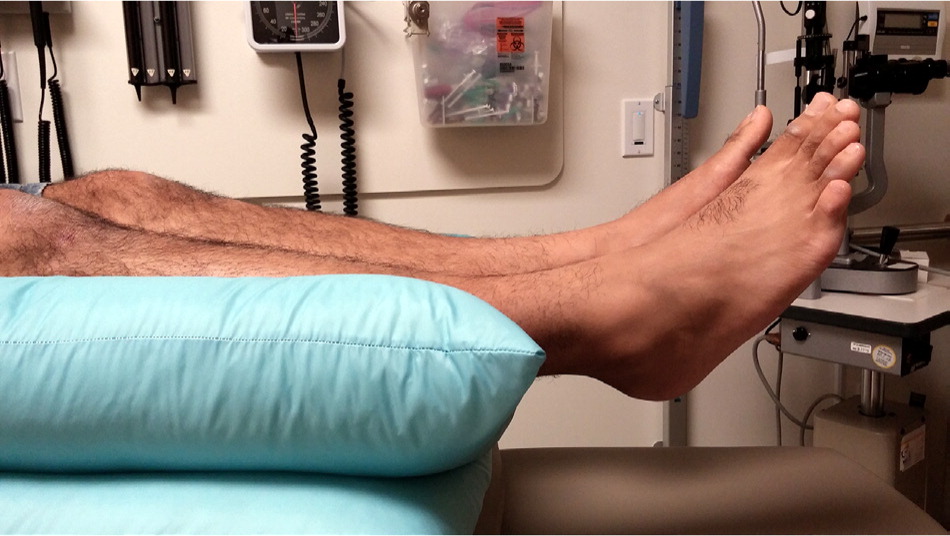
Three systematic reviews found moderate-strength evidence supporting the use of air-fluidized beds, radiant heat dressings (a noncontact dressing attached to a heating element that provides warmth to increase capillary blood flow and resistance to infection), and electrical stimulation (direct electric current delivered through the wound bed using surface electrodes) to improve pressure ulcer healing.4,22,30 Electrical stimulation is specifically recommended in the American College of Physicians guidelines.22 With electrical stimulation, high-voltage pulse current should be used because it has been better studied, penetrates deeper, and has a lower risk of burns. Daily one-hour sessions are commonly used. Most studies used stimulus pulse settings of a 50-microsecond duration, approximately 100 Hz in frequency, and 100 to 150 V in intensity.30 Electrical stimulation should not be used in patients with cancer because it could stimulate cancerous cells, or in patients with osteomyelitis because it may cause premature closure.30
Low-strength evidence supports the use of alternating-pressure surfaces, platelet-derived growth factor, and light therapy (infrared, visible, and ultraviolet spectrum light).4,22 However, a Cochrane review concluded that because of the low-quality evidence, the effects of phototherapy are uncertain.31
If consistent with a patient's goals of care, surgical consultation is recommended based on expert opinion and usual practice for stage 3 or 4 ulcers not responding to standard therapy. There is insufficient evidence to support one surgical technique over another, and dehiscence is common.22
When Should Osteomyelitis or Other Infectious Complications Be Considered?
The decision to treat a pressure ulcer for infection should be a clinical one. Ideally, evidence of infection is confirmed by tissue or bone biopsy. Superficial cultures usually are not useful other than to rule out methicillin-resistant Staphylococcus aureus (MRSA) colonization. Systemic rather than topical antibiotics should be used to treat an infected pressure ulcer.1,32
EVIDENCE SUMMARY
The diagnosis of wound infection is usually a clinical determination. Although tissue or bone biopsy has long been considered the preferred method to identify infection or osteomyelitis, this is often not practical in the clinical setting.1,32 All open pressure ulcers contain bacteria, which, unless they reach high colony counts, should not impair wound healing.23 Therefore, culture is not usually helpful to determine infection, other than to rule out the presence of MRSA. If a superficial culture is taken, evidence suggests that the Levine technique should be used, which involves a swab rotated over a 1-cm square patch of wound with enough pressure to extract fluid from the wound for five seconds.32 When bone is exposed in a pressure ulcer, osteomyelitis can be clinically presumed.
Of all clinical signs of infection, a systematic review suggests that worsening pain (in patients with intact sensation) may be one of the best predictors of chronic wound infection.32 Other signs of an acute spreading infection may include erythema around the ulcer's edges, induration, warmth, purulent drainage, or worsening of chronic drainage. Additional signs of infection include no evidence of healing for two weeks, friable granulation tissue, foul odor, new necrotic tissue, or lack of uniform spread of granulation tissue across the base of the wound (referred to as pocketing or bridging). Systemic signs may include fever, lymph node enlargement, or possible delirium or confusion.1
Pressure ulcers that are large, have necrotic tissue or a foreign body, have been present a long time, or are repeatedly contaminated by stool are at greater risk of becoming infected. Patients at higher risk of infection are also at higher risk of developing ulcers initially, including patients with diabetes mellitus, undernutrition, poor tissue perfusion, or immune suppression.
To identify osteomyelitis on imaging, radiography is often used first, although magnetic resonance imaging and nuclear medicine tests are more sensitive and specific than conventional plain radiography.33 Systemic antibiotics should be used to treat infected ulcers. When treating osteomyelitis, infectious disease consultation, if available, should be considered to help tailor antibiotic therapy. There is little role for the use of topical antibiotics.1
Data Sources: A search was performed using Essential Evidence Plus. The term pressure ulcer was searched in the Cochrane Database of Systematic Reviews and the evidence-based guidelines from the National Guideline Clearinghouse. Additionally, a PubMed search of systematic reviews was done using the search term pressure ulcer. Search dates: January 2014 through July 2015.
The authors thank Elliot Twiggs, MD, for his assistance with Figure 3.
