
A more recent article on newborn respiratory distress is available.
Am Fam Physician. 2015;92(11):994-1002
Author disclosure: No relevant financial affiliations.
Newborn respiratory distress presents a diagnostic and management challenge. Newborns with respiratory distress commonly exhibit tachypnea with a respiratory rate of more than 60 respirations per minute. They may present with grunting, retractions, nasal flaring, and cyanosis. Common causes include transient tachypnea of the newborn, respiratory distress syndrome, meconium aspiration syndrome, pneumonia, sepsis, pneumothorax, persistent pulmonary hypertension of the newborn, and delayed transition. Congenital heart defects, airway malformations, and inborn errors of metabolism are less common etiologies. Clinicians should be familiar with updated neonatal resuscitation guidelines. Initial evaluation includes a detailed history and physical examination. The clinician should monitor vital signs and measure oxygen saturation with pulse oximetry, and blood gas measurement may be considered. Chest radiography is helpful in the diagnosis. Blood cultures, serial complete blood counts, and C-reactive protein measurement are useful for the evaluation of sepsis. Most neonates with respiratory distress can be treated with respiratory support and noninvasive methods. Oxygen can be provided via bag/mask, nasal cannula, oxygen hood, and nasal continuous positive airway pressure. Ventilator support may be used in more severe cases. Surfactant is increasingly used for respiratory distress syndrome. Using the INSURE technique, the newborn is intubated, given surfactant, and quickly extubated to nasal continuous positive airway pressure. Newborns should be screened for critical congenital heart defects via pulse oximetry after 24 hours but before hospital discharge. Neonatology consultation is recommended if the illness exceeds the clinician's expertise and comfort level or when the diagnosis is unclear in a critically ill newborn.
Newborn respiratory distress occurs in about 7% of deliveries.1 Respiratory distress syndrome, which occurs primarily in premature infants, affects about 1% of newborns, resulting in about 860 deaths per year.2 With increased survival of preterm and late preterm infants, management of respiratory distress in newborns has become challenging.3,4 Because early recognition improves the care of these newborns, clinicians must be familiar with its diagnosis and treatment.
| Clinical recommendation | Evidence rating | References | Comments |
|---|---|---|---|
| Noninvasive ventilation, commonly using nasal continuous positive airway pressure, may replace invasive intubation because of improved clinical and financial outcomes. | B | 15 | Randomized controlled trial |
| The minimum required amount of surfactant is 100 mg per kg. Initial administration of 200 mg per kg can result in significant improvement in oxygenation and decreased need to retreat. | B | 17, 18 | Randomized controlled trials |
| The INSURE (intubate, administer surfactant, extubate to nasal continuous positive airway pressure) strategy should be used to reduce mechanical ventilation, air leak syndromes, and progression to bronchopulmonary dysplasia. | B | 19 | Cochrane review |
| Antenatal corticosteroids given between 24 and 34 weeks' gestation decrease respiratory distress syndrome risk with a number needed to treat of 11. | C | 6 | Consensus guidelines |
| The U.S. Department of Health and Human Services recommends screening newborns for critical congenital heart defects using pulse oximetry before hospital discharge, but at least 24 hours after birth. | C | 53 | Prospective study |
WHAT IS NEW ON THIS TOPIC: NEWBORN RESPIRATORY DISTRESS
The U.S. Department of Health and Human Services recommends routine pulse oximetry over physical examination alone as a screening strategy for critical congenital heart disease.
Maternal selective serotonin reuptake inhibitor use late in pregnancy is associated with a small absolute increased risk for persistent pulmonary hypertension of the newborn.
Reduction of premature births and cesarean deliveries decreases respiratory distress cases, with prenatal care being crucial to prevention. Women with inadequate prenatal care may deliver babies with lower birth weights and increased risk of admission to the neonatal intensive care unit.5 Antenatal corticosteroid use in threatened preterm deliveries from 24 to 34 weeks' gestation significantly reduces the incidence and severity of respiratory distress.6 Because cesarean delivery is a risk factor for respiratory distress, especially in premature infants, reducing these surgeries when possible could reduce the incidence of the condition.7
Clinical Presentation
Tachypnea is the most common presentation in newborns with respiratory distress. A normal respiratory rate is 40 to 60 respirations per minute. Other signs may include nasal flaring, grunting, intercostal or subcostal retractions, and cyanosis. The newborn may also have lethargy, poor feeding, hypothermia, and hypoglycemia.
The most common causes of respiratory distress in newborns are transient tachypnea of the newborn (TTN), respiratory distress syndrome (RDS), meconium aspiration syndrome, pneumonia, sepsis, pneumothorax, and delayed transition. Rare causes include choanal atresia; diaphragmatic hernia; tracheoesophageal fistula; congenital heart disease; and neurologic, metabolic, and hematologic disorders. The differential diagnosis of newborn respiratory distress is listed in Table 1.8
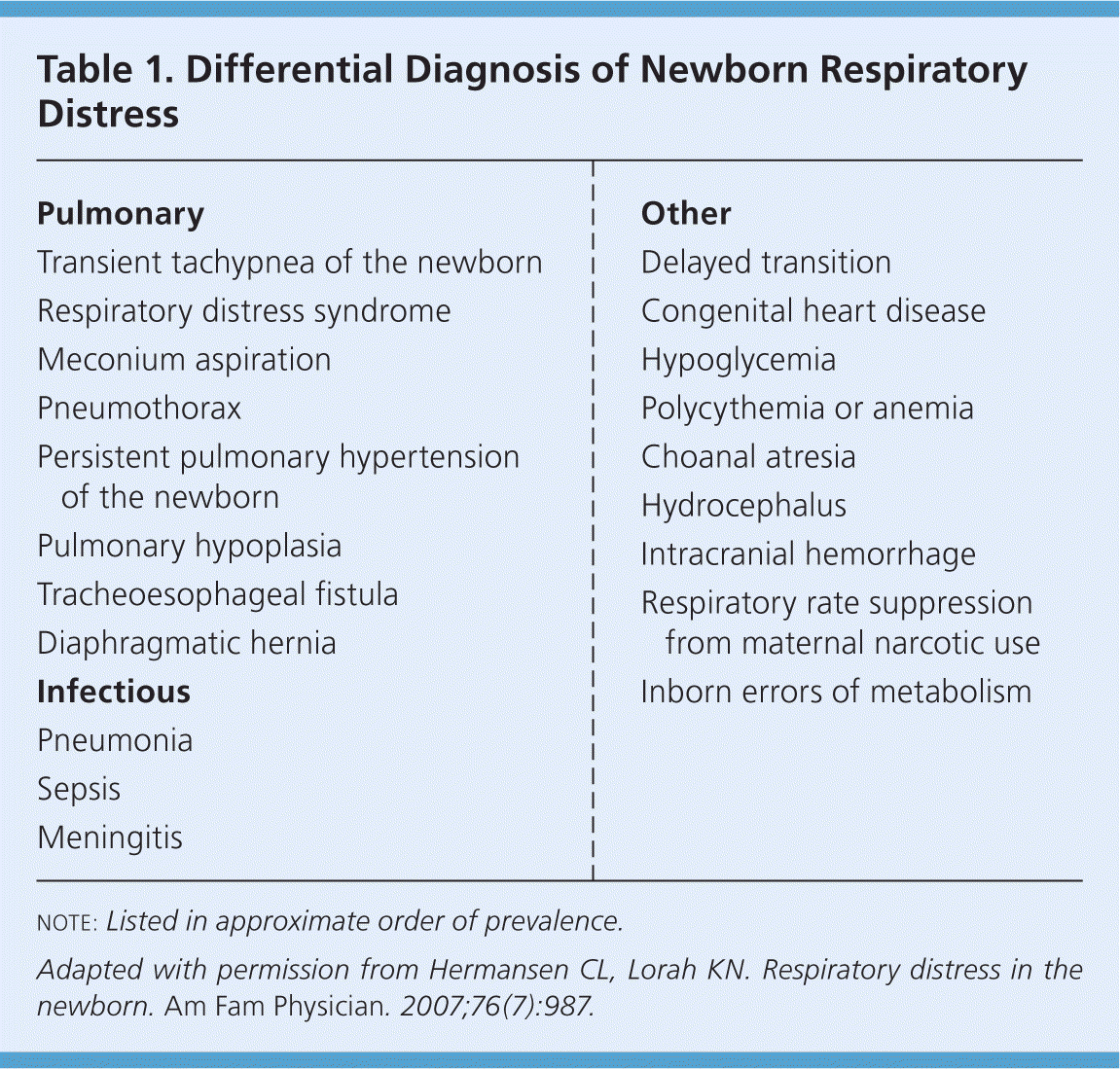
| Pulmonary |
| Transient tachypnea of the newborn |
| Respiratory distress syndrome |
| Meconium aspiration |
| Pneumothorax |
| Persistent pulmonary hypertension of the newborn |
| Pulmonary hypoplasia |
| Tracheoesophageal fistula |
| Diaphragmatic hernia |
| Infectious |
| Pneumonia |
| Sepsis |
| Meningitis |
| Other |
| Delayed transition |
| Congenital heart disease |
| Hypoglycemia |
| Polycythemia or anemia |
| Choanal atresia |
| Hydrocephalus |
| Intracranial hemorrhage |
| Respiratory rate suppression from maternal narcotic use |
| Inborn errors of metabolism |
Rarely, newborns with RDS develop chronic lung disease or bronchopulmonary dysplasia. Definitions have been established for bronchopulmonary dysplasia severity (Table 2).9 Newborns with bronchopulmonary dysplasia may have nutritional failure, have neurodevelopmental delays, and require oxygen for a longer period with higher hospital readmission rates.10
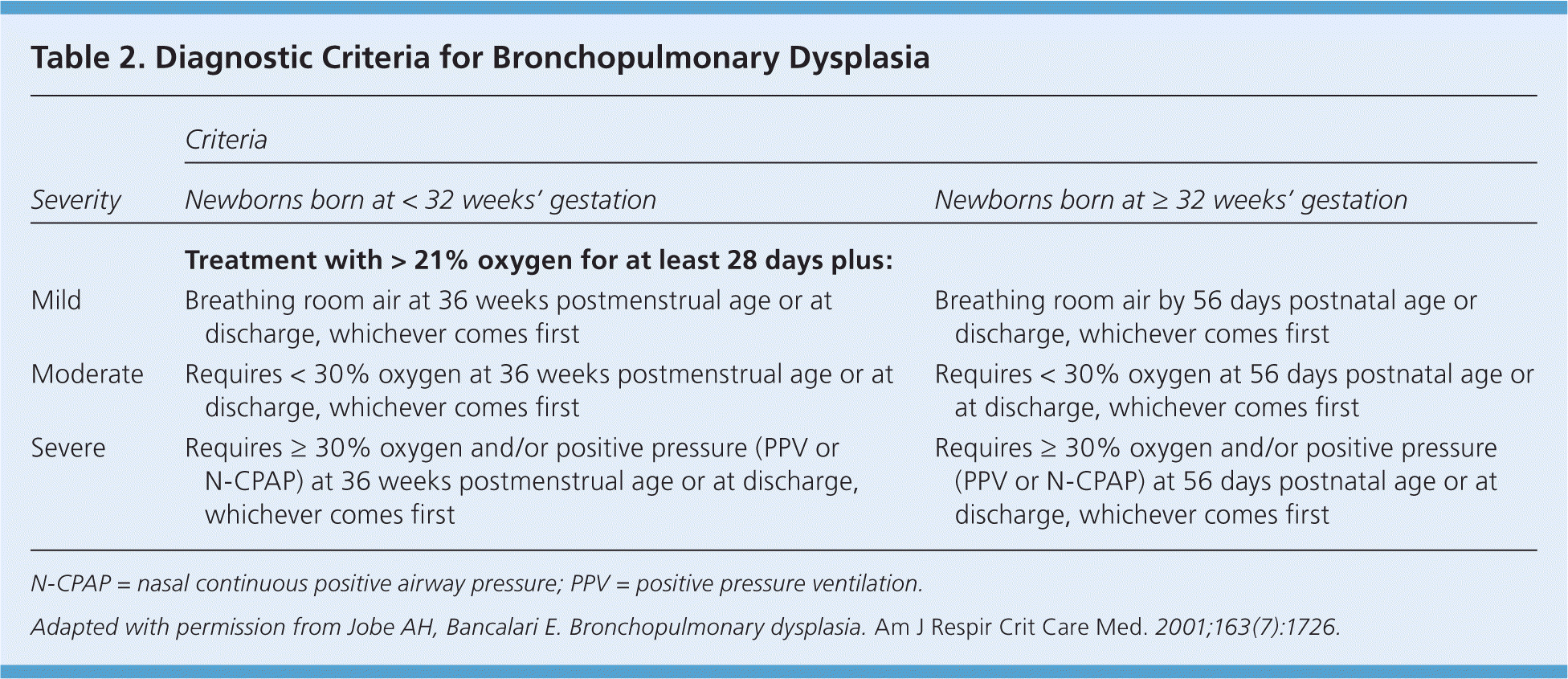
| Severity | Criteria | |
|---|---|---|
| Newborns born at < 32 weeks' gestation | Newborns born at ≥ 32 weeks' gestation | |
| Treatment with > 21% oxygen for at least 28 days plus: | ||
| Mild | Breathing room air at 36 weeks postmenstrual age or at discharge, whichever comes first | Breathing room air by 56 days postnatal age or discharge, whichever comes first |
| Moderate | Requires < 30% oxygen at 36 weeks postmenstrual age or at discharge, whichever comes first | Requires < 30% oxygen at 56 days postnatal age or at discharge, whichever comes first |
| Severe | Requires ≥ 30% oxygen and/or positive pressure (PPV or N-CPAP) at 36 weeks postmenstrual age or at discharge, whichever comes first | Requires ≥ 30% oxygen and/or positive pressure (PPV or N-CPAP) at 56 days postnatal age or at discharge, whichever comes first |
Diagnosis

| Test | Comments |
|---|---|
| Blood culture |
|
| Blood gas |
|
| Blood glucose |
|
| Chest radiography |
|
| Complete blood count with differential |
|
| C-reactive protein |
|
| Pulse oximetry |
|
Laboratory data can assist in the diagnosis. Complete blood counts with an immature to total neutrophil ratio of more than 0.2 is suggestive of infection. This ratio can be altered by stress, crying, and labor induced with oxytocin (Pitocin).11 Although the immature to total neutrophil ratio has significant sensitivity and negative predictive value, it has poor positive predictive accuracy as a one-time test and is falsely elevated in 50% of infants without an infection.11 C-reactive protein levels of less than 10 mg per L (95.24 nmol per L) rule out sepsis with a 94% negative predictive value when obtained 24 and 48 hours after birth.12 Glucose levels should also be measured because hypoglycemia can be a cause and consequence of respiratory distress.
General Treatment Principles
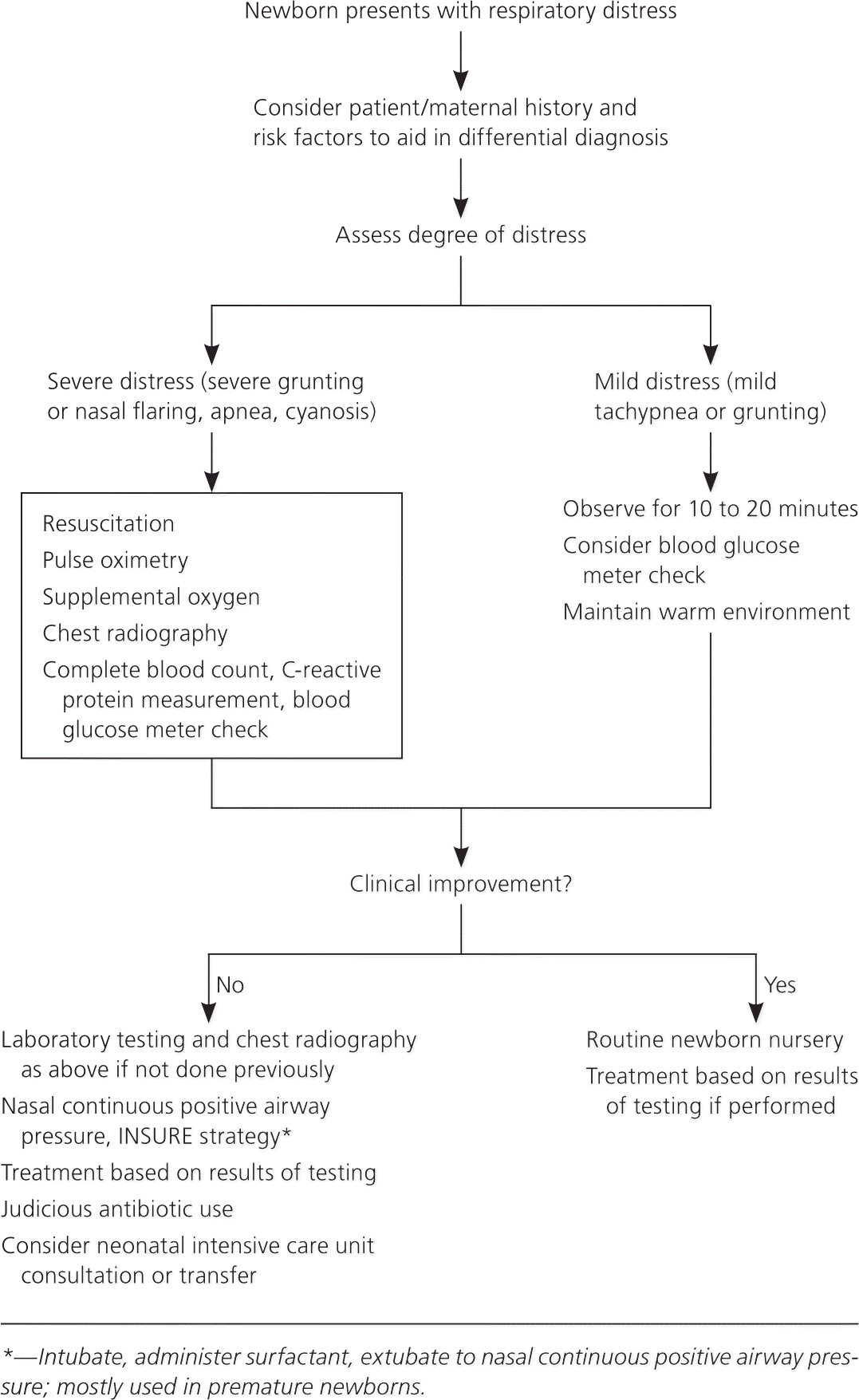
RESPIRATORY SUPPORT
Oxygenation can be maintained by delivering oxygen via bag/mask, nasal cannula, oxygen hood, nasal continuous positive airway pressure (N-CPAP), or ventilator support. Resuscitation with 100% oxygen may increase neonatal mortality compared with ambient air.13 Blended oxygen, with the fraction of inspired oxygen ranging from 21% to 50% oxygen, stabilizes premature newborns, and pulse oximetry monitors are used to maintain saturations around 90%.14
Noninvasive ventilation, commonly using N-CPAP, has become the standard respiratory treatment over invasive intubation. N-CPAP has decreased transfers to tertiary care centers with a number needed to treat of 7.3 and a potential cost reduction of $10,000 per case.15 Nasal intermittent positive pressure ventilation can also be used. In preterm newborns with RDS, nasal intermittent positive pressure ventilation has been shown to reduce the relative need for mechanical ventilation by 60%.16 Conventional mechanical ventilation is reserved for more severe cases.
SURFACTANT REPLACEMENT
Prophylactic and rescue therapy with natural surfactants in newborns with RDS reduces air leaks and mortality. Neonatal type II pneumocytes produce surfactant in the third trimester to prepare for air breathing. Without surfactant, there is higher pulmonary surface tension, atelectasis, and ventilation/perfusion mismatch resulting in hypoxia, hypercapnia, and acidosis.
The minimum required amount of surfactant therapy is 100 mg per kg. An initial dose of 200 mg per kg leads to a statistically significant improvement in oxygenation and decreased need to retreat, although there is no survival benefit.17,18 A Cochrane review showed that the technique known as INSURE (intubate, administer surfactant, extubate to N-CPAP) led to a 67% relative risk reduction for mechanical ventilation and about a 50% relative risk reduction for air leak syndromes and progression to bronchopulmonary dysplasia.19 The American Academy of Pediatrics recently released guidelines for surfactant use in newborns with respiratory distress.20
OTHER SUPPORT
Adequate fluid and electrolyte balance should be maintained. Oral feedings are withheld if the respiratory rate exceeds 60 respirations per minute to prevent aspiration. A neutral thermal environment reduces the newborn's energy requirements and oxygen consumption.21 If the illness exceeds the clinician's expertise and comfort level or the diagnosis is unclear in a critically ill newborn, neonatology should be consulted.
Etiologies
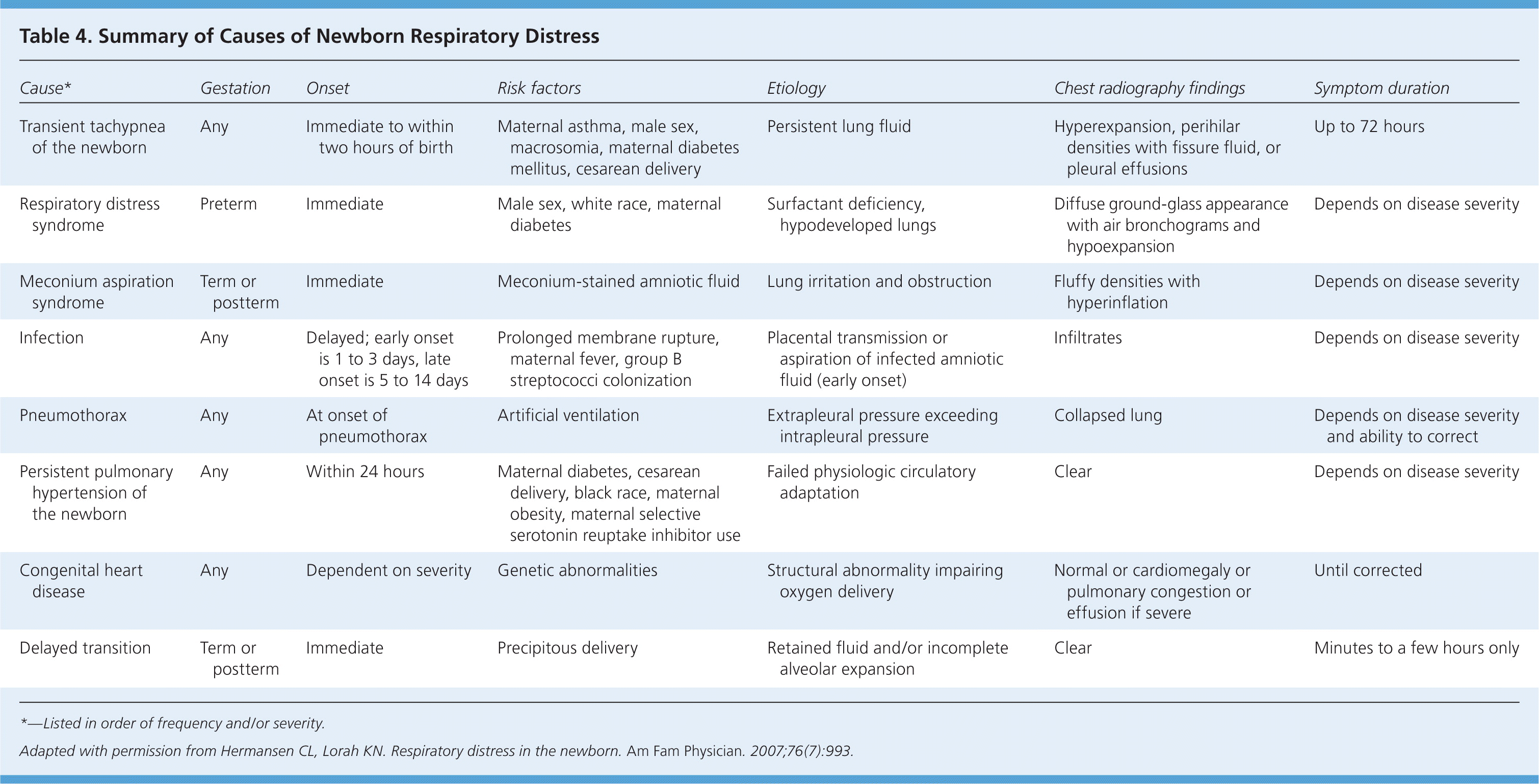
| Cause* | Gestation | Onset | Risk factors | Etiology | Chest radiography findings | Symptom duration |
|---|---|---|---|---|---|---|
| Transient tachypnea of the newborn | Any | Immediate to within two hours of birth | Maternal asthma, male sex, macrosomia, maternal diabetes mellitus, cesarean delivery | Persistent lung fluid | Hyperexpansion, perihilar densities with fissure fluid, or pleural effusions | Up to 72 hours |
| Respiratory distress syndrome | Preterm | Immediate | Male sex, white race, maternal diabetes | Surfactant deficiency, hypodeveloped lungs | Diffuse ground-glass appearance with air bronchograms and hypoexpansion | Depends on disease severity |
| Meconium aspiration syndrome | Term or postterm | Immediate | Meconium-stained amniotic fluid | Lung irritation and obstruction | Fluffy densities with hyperinflation | Depends on disease severity |
| Infection | Any | Delayed; early onset is 1 to 3 days, late onset is 5 to 14 days | Prolonged membrane rupture, maternal fever, group B streptococci colonization | Placental transmission or aspiration of infected amniotic fluid (early onset) | Infiltrates | Depends on disease severity |
| Pneumothorax | Any | At onset of pneumothorax | Artificial ventilation | Extrapleural pressure exceeding intrapleural pressure | Collapsed lung | Depends on disease severity and ability to correct |
| Persistent pulmonary hypertension of the newborn | Any | Within 24 hours | Maternal diabetes, cesarean delivery, black race, maternal obesity, maternal selective serotonin reuptake inhibitor use | Failed physiologic circulatory adaptation | Clear | Depends on disease severity |
| Congenital heart disease | Any | Dependent on severity | Genetic abnormalities | Structural abnormality impairing oxygen delivery | Normal or cardiomegaly or pulmonary congestion or effusion if severe | Until corrected |
| Delayed transition | Term or postterm | Immediate | Precipitous delivery | Retained fluid and/or incomplete alveolar expansion | Clear | Minutes to a few hours only |
TRANSIENT TACHYPNEA OF THE NEWBORN
The most common etiology of respiratory distress in newborns is TTN, which occurs in about five or six per 1,000 births.22 It is more common in newborns of mothers with asthma.23 Newborns with TTN have a greater risk of developing asthma in childhood; in one study, this association was stronger in patients of lower socioeconomic status, nonwhite race, and males whose mothers did not have asthma.24 TTN results from delayed reabsorption and clearance of alveolar fluid. Postdelivery prostaglandin release distends lymphatic vessels, which removes lung fluid as pulmonary circulation increases with the initial fetal breath. Cesarean delivery without labor bypasses this process and is therefore a risk factor for TTN.25 Surfactant deficiency may play a role in TTN. Research indicates a decreased count of lamellar bodies in the gastric aspirate and decreased surfactant phospholipid concentrations in the tracheal aspirate in cases of TTN. However, treating TTN with surfactant is not indicated.26,27
TTN presents within two hours of birth and can persist for 72 hours. Breath sounds can be clear or reveal rales on auscultation. The higher the respiratory rate at onset, the longer TTN is likely to last.28,29 Chest radiography findings (Figure 230 ) support a clinical diagnosis, revealing hyperexpansion, perihilar densities with fissure fluid, or pleural effusions. Blood gases may show hypoxemia, hypercapnia, or respiratory acidosis.
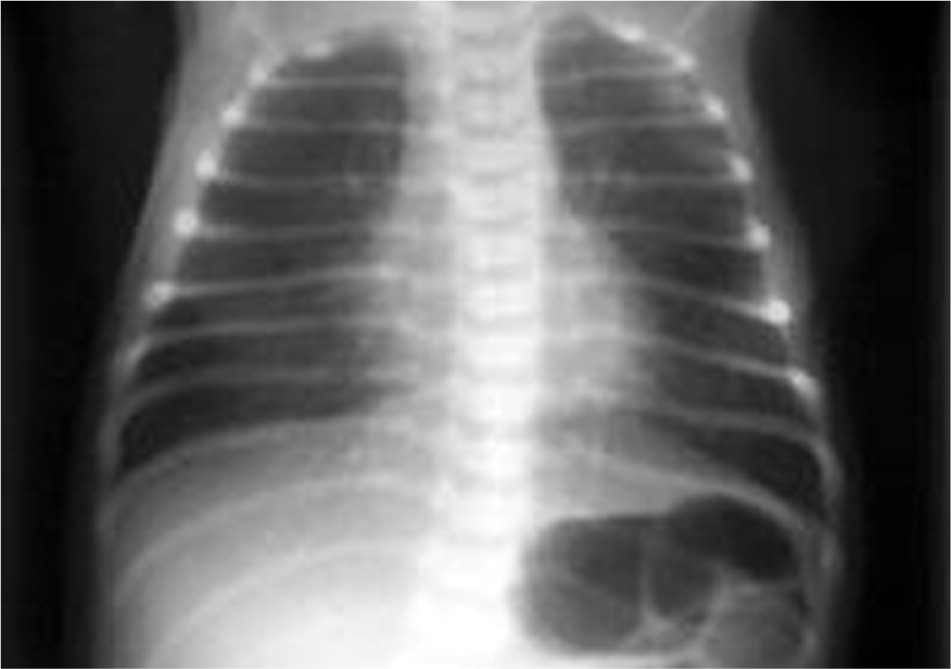
Because TTN is self-limited, treatment is supportive. Furosemide (Lasix) may cause weight loss and hyponatremia, and it is contraindicated despite the excess pulmonary fluid present in newborns with TTN.31 Fluid restriction in TTN is beneficial, reducing the duration of respiratory support and hospital-related costs.32 Inhaled albuterol reduces tachypnea duration and the need for oxygen therapy, although standardized guidelines are still needed.33 Antibiotics are not indicated in TTN.34 Antenatal corticosteroids given 48 hours before elective cesarean delivery at 37 to 39 weeks' gestation reduce TTN incidence, although it is unclear whether delaying cesarean delivery until 39 weeks' gestation is preferable.6
RESPIRATORY DISTRESS SYNDROME
RDS symptoms (i.e., tachypnea, grunting, retractions, and cyanosis) occur immediately after birth. Chest radiography (Figure 337 ) shows a diffuse ground-glass appearance with air bronchograms and hypoexpansion, and blood gas measurements show hypoxemia and acidosis. Symptoms normally worsen in the first 12 to 24 hours. With advances in treatment such as surfactant and N-CPAP, most newborns with RDS recover without long-term effects. Bronchopulmonary dysplasia can occur in complicated cases, leading to recurrent wheezing, asthma, and higher hospital admission rates later in life.38
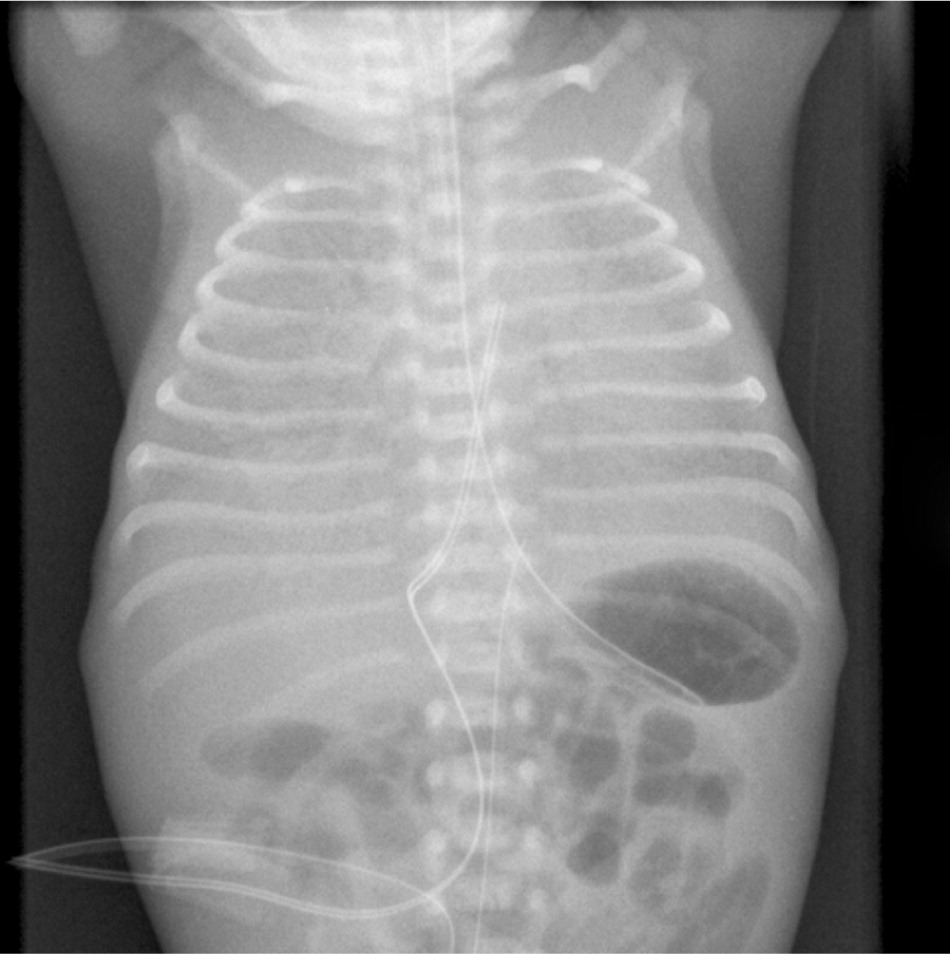
Antenatal corticosteroids given between 24 and 34 weeks' gestation decrease RDS risk with a number needed to treat of 11.39 A single dose of antenatal corticosteroids is beneficial if given more than 24 hours before delivery and provides coverage for seven days. The use of repetitive antenatal corticosteroid doses to prevent RDS is debatable, but no more than two courses are recommended.40
MECONIUM ASPIRATION SYNDROME
Meconium-stained amniotic fluid is present in approximately 10% to 15% of deliveries, although the incidence of meconium aspiration syndrome is only 1%.41,42 Because meconium excretion often represents fetal maturity, meconium aspiration syndrome occurs in term and post-term newborns. Meconium is a conglomeration of desquamated cells, bile pigments, pancreatic enzymes, and amniotic fluid. Although sterile, it can lead to bacterial infection, irritation, obstruction, and pneumonia.
Meconium aspiration syndrome presents at birth as marked tachypnea, grunting, retractions, and cyanosis. Examination may reveal a barrel-shaped chest, with rales and rhonchi heard on auscultation. Chest radiography (Figure 437 ) may show bilateral fluffy densities with hyperinflation. Treatment includes N-CPAP and supplemental oxygen. Ventilator support may be needed in more severe cases.
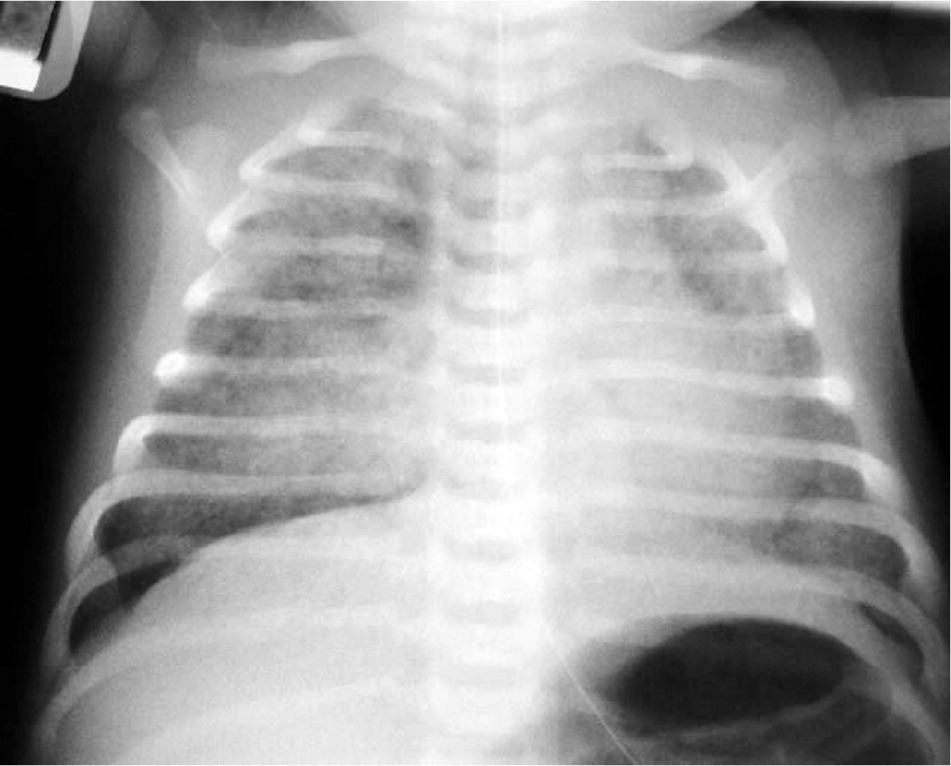
Perineal neonatal suctioning for meconium does not prevent aspiration. If the infant is hypotonic at birth, intubation and meconium suctioning are advised. Vigorous infants receive expectant management.43
NEONATAL SEPSIS AND PNEUMONIA
Sepsis can occur in full-term and preterm infants and has an incidence of one or two per 1,000 live births.44 Symptoms may begin later in the newborn period. Risk factors include membrane rupture more than 18 hours before delivery, prematurity, and maternal fever. Common pathogens include group B streptococci, Escherichia coli, Listeria monocytogenes, Haemophilus influenzae, Staphylococcus aureus, and gram-negative organisms. Universal screening and antepartum antibiotics for group B streptococci carriers reduce early-onset disease.45 However, 5,701 patients need to be screened and 1,191 patients treated to prevent one infection.46 A risk calculator can be used to estimate the probability of neonatal early-onset infection.47
Early-onset pneumonia occurs within the first three days of life, resulting from placental transmission of bacteria or aspiration of infected amniotic fluid. Late-onset pneumonia occurs after hospital discharge. Bacterial pathogens are similar to those that cause sepsis.
Serial complete blood counts, C-reactive protein measurements, and blood cultures help with diagnosis and treatment. Intravenous antibiotics are administered if bacterial infection is suspected. Ampicillin and gentamicin are common antibiotics for early-onset infections, whereas vancomycin and/or oxacillin with an aminoglycoside are used for late-onset infections. Antibiotics should be used judiciously.48 Treatment duration depends on clinical condition and laboratory findings.
PNEUMOTHORAX
Pneumothorax occurs if pulmonary space pressure exceeds extrapleural pressure, either spontaneously or secondary to an infection, aspiration, lung deformity, or ventilation barotrauma. Spontaneous pneumothorax occurs in 1% to 2% of term births, and more often in premature births and in newborns with RDS or meconium aspiration syndrome.49 A small pneumothorax may be asymptomatic.
Although transillumination can be helpful, chest radiography confirms the diagnosis. Symptomatic newborns need supplemental oxygen. Tension pneumothorax requires immediate needle decompression or chest tube drainage.
PERSISTENT PULMONARY HYPERTENSION OF THE NEWBORN
Delicate physiologic mechanisms allow for circulatory transition after birth with a resultant decrease in pulmonary vascular resistance. Failure of these mechanisms causes increased pulmonary pressures and right-to-left shunting, resulting in hypoxemia. This failure can be caused by meconium aspiration syndrome, pneumonia or sepsis, severe RDS, diaphragmatic hernia, and pulmonary hypoplasia. Severe persistent pulmonary hypertension of the newborn (PPHN) occurs in two out of 1,000 live births.50 Risk factors include maternal diabetes, cesarean delivery, maternal obesity, and black race. Maternal use of a selective serotonin reuptake inhibitor is associated with the condition. Data show only a small absolute risk.51
With PPHN, respiratory distress occurs within 24 hours of birth. On examination, a loud second heart sound and systolic murmur may be heard. Oxygen saturation or PaO2 increases when 100% oxygen is provided. Echocardiography should be performed to confirm the diagnosis. PPHN is treated with oxygen and other support. In serious cases, ventilator or vasopressor support and/or use of pulmonary vasodilators such as inhaled nitric oxide or sildenafil (Revatio) may be helpful. A few cases require extracorporeal membrane oxygenation.
CONGENITAL HEART DEFECTS
Congenital heart defects occur in about 1% of births in the United States annually. One-fourth of cases are critical, necessitating surgery in the first year, and one-fourth of those newborns do not survive the first year.52 Newborns with cyanotic heart disease present with intense cyanosis that is disproportionate to respiratory distress. Cardiac murmur may be heard on examination. Decreases in femoral pulses and lower extremity blood pressures may indicate coarctation of the aorta. Providing 100% oxygen will not improve oxygen saturation. Chest radiography and electrocardiography may indicate congenital structural abnormalities, and echocardiography can confirm the diagnosis.
The U.S. Department of Health and Human Services recommends pulse oximetry over physical examination alone to screen for critical congenital heart defects.53 Newborns should be screened before hospital discharge, but at least 24 hours after birth. The cost of treating one critical congenital heart defect exceeds the cost of screening more than 2,000 newborns, with 20 infant deaths prevented with screening.54,55 Pulse oximetry screening for critical congenital heart defects is becoming standard practice before hospital discharge.
DELAYED TRANSITION
The diagnosis of delayed transition is made retrospectively when symptoms cease without another identified etiology. It results from retained fluid and incompletely expanded alveoli from a precipitous vaginal delivery, as pathophysiologic mechanisms have not had sufficient time to adjust to extrauterine life. Treatment is supportive until the distress resolves a few hours after transition concludes.
| Case Studies | |
|---|---|
| CASE 1 | CASE 2 |
|
|
Data Sources: A PubMed search was completed in Clinical Queries using the key terms newborn, distress, respiratory, meconium, and tachypnea. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. Also searched were DynaMed, Clinical Evidence, the Cochrane database, Essential Evidence Plus, the National Guideline Clearinghouse database, and the American Academy of Pediatrics. Search dates: October 2014 to March 2015.
