
Am Fam Physician. 2016;93(12):1008-1010
Author disclosure: No relevant financial affiliations.
Clinical Question
Are new high-sensitivity cardiac troponin tests accurate enough to rule out myocardial infarction within two hours of a patient's arrival in the emergency department (ED)?
Evidence Summary
Cardiac troponin T and I are released into the bloodstream when cardiac muscle is damaged. Cardiac troponin tests have been available for decades and are the preferred biomarkers for the diagnosis of acute myocardial infarction (AMI).1 However, until recently, they lacked sensitivity in the first few hours following an acute myocardial injury. Therefore, AMI could not be ruled out until the patient had a normal troponin test result at least six hours after the onset of chest pain.2
Recently, several companies have developed novel high-sensitivity troponin assays. They include a high-sensitivity troponin T (hsTnT) assay and a high-sensitivity troponin I (hsTnI) assay. Because the interpretation of tests differs, it is important for physicians to know which test is being used at their institution and the test's lowest detectable level.
Researchers have begun to explore protocols with the potential to rule out AMI earlier, so that patients can be discharged within a few hours of arrival rather than six to 12, or even 24, hours later. These protocols have used hsTnT3–6 and hsTnI.7,8 There are two general approaches. The first identifies the lowest identifiable level of troponin, and AMI is ruled out in any patient with an undetectable level of troponin on arrival at the ED.3,7,8 The second identifies patients with an initial low troponin level and no more than a small increase in levels between arrival at the ED and a second measurement one to two hours later.4,5 All of these protocols excluded patients with an obvious cause of chest pain such as vehicular trauma or who had ischemic changes on electrocardiography (ECG).
To be useful, a test should identify a substantial group of patients with a very low likelihood of AMI or cardiovascular death in the next 30 days, to allow outpatient evaluation of the patient's chest pain. Protocols that rule out a larger percentage of patients are preferable to those that rule out only a few. Table 1 summarizes the most recent evidence regarding protocols to rule out AMI, by type of test and the percentage of patients in each study meeting the rule-out criteria.3–8 Studies ranged in size from 270 to 14,636 patients and evaluated the ability to rule out AMI and determine 30-day mortality. Among protocols using the hsTnI assay, the most useful used a rule-out criteria of no AMI on the initial ECG and an initial hsTnI level of less than 5 ng per L; 46.1% of patients met these rule-out criteria.7 The study was large, with more than 6,000 patients, and only 0.4% (one in 250) had an AMI or cardiac death in the 30 days after discharge from the ED.
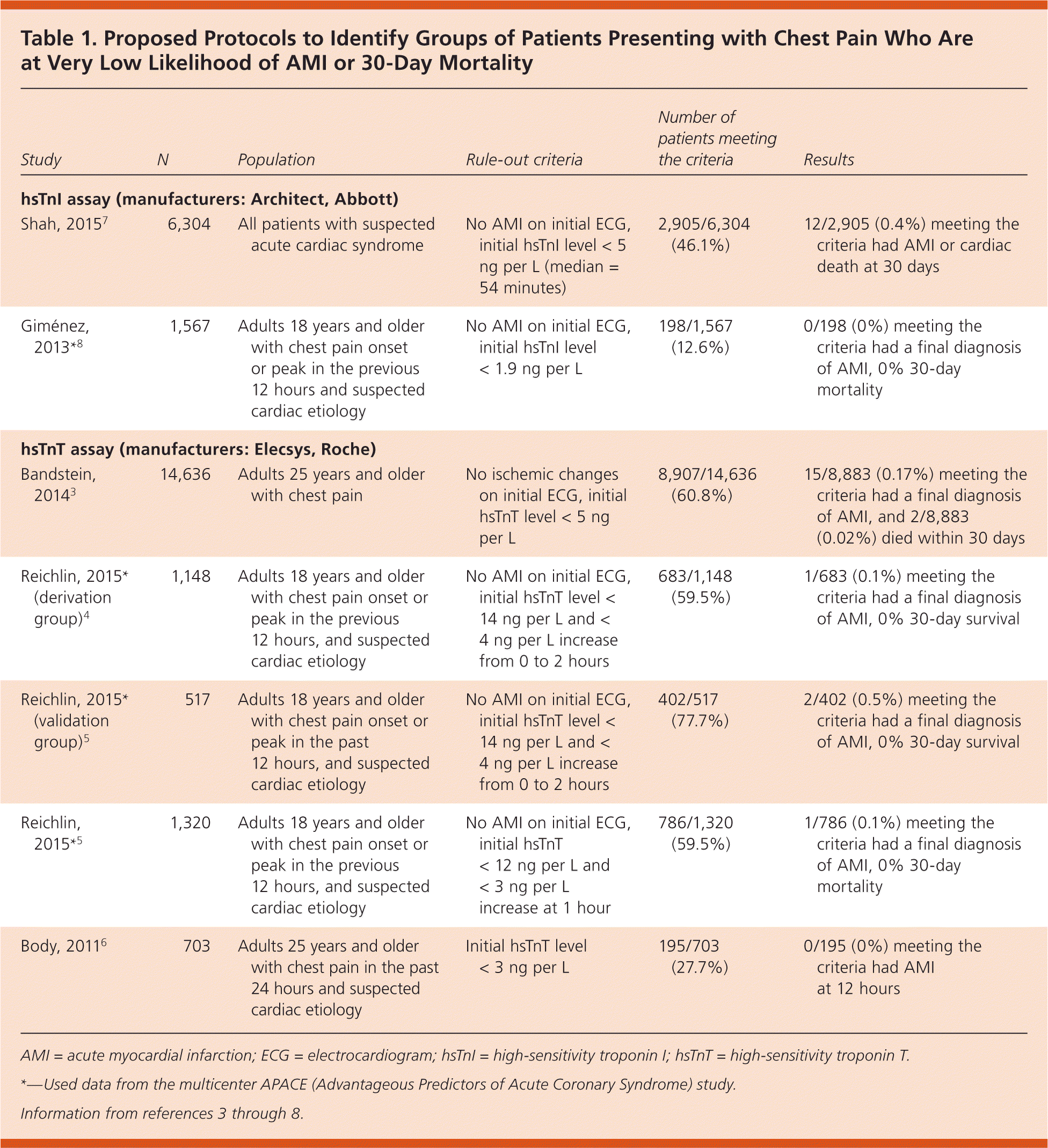
| Study | N | Population | Rule-out criteria | Number of patients meeting the criteria | Results |
|---|---|---|---|---|---|
| hsTnI assay (manufacturers: Architect, Abbott) | |||||
| Shah, 20157 | 6,304 | All patients with suspected acute cardiac syndrome | No AMI on initial ECG, initial hsTnI level < 5 ng per L (median = 54 minutes) | 2,905/6,304 (46.1%) | 12/2,905 (0.4%) meeting the criteria had AMI or cardiac death at 30 days |
| Giménez, 2013*8 | 1,567 | Adults 18 years and older with chest pain onset or peak in the previous 12 hours and suspected cardiac etiology | No AMI on initial ECG, initial hsTnI level < 1.9 ng per L | 198/1,567 (12.6%) | 0/198 (0%) meeting the criteria had a final diagnosis of AMI, 0% 30-day mortality |
| hsTnT assay (manufacturers: Elecsys, Roche) | |||||
| Bandstein, 20143 | 14,636 | Adults 25 years and older with chest pain | No ischemic changes on initial ECG, initial hsTnT level < 5 ng per L | 8,907/14,636 (60.8%) | 15/8,883 (0.17%) meeting the criteria had a final diagnosis of AMI, and 2/8,883 (0.02%) died within 30 days |
| Reichlin, 2015* (derivation group)4 | 1,148 | Adults 18 years and older with chest pain onset or peak in the previous 12 hours, and suspected cardiac etiology | No AMI on initial ECG, initial hsTnT level < 14 ng per L and < 4 ng per L increase from 0 to 2 hours | 683/1,148 (59.5%) | 1/683 (0.1%) meeting the criteria had a final diagnosis of AMI, 0% 30-day survival |
| Reichlin, 2015* (validation group)5 | 517 | Adults 18 years and older with chest pain onset or peak in the past 12 hours, and suspected cardiac etiology | No AMI on initial ECG, initial hsTnT level < 14 ng per L and < 4 ng per L increase from 0 to 2 hours | 402/517 (77.7%) | 2/402 (0.5%) meeting the criteria had a final diagnosis of AMI, 0% 30-day survival |
| Reichlin, 2015*5 | 1,320 | Adults 18 years and older with chest pain onset or peak in the previous 12 hours, and suspected cardiac etiology | No AMI on initial ECG, initial hsTnT < 12 ng per L and < 3 ng per L increase at 1 hour | 786/1,320 (59.5%) | 1/786 (0.1%) meeting the criteria had a final diagnosis of AMI, 0% 30-day mortality |
| Body, 20116 | 703 | Adults 25 years and older with chest pain in the past 24 hours and suspected cardiac etiology | Initial hsTnT level < 3 ng per L | 195/703 (27.7%) | 0/195 (0%) meeting the criteria had AMI at 12 hours |
Among protocols using the hsTnT assay, the one identifying the largest percentage of patients as low risk used both a low initial hsTnT and no more than a small increase in that value over the next one to two hours.4,5 These were defined as an initial troponin T value less than 14 ng per L that increases by less than 4 ng per L in the next two hours, or an initial level less than 13 ng per L that increases by less than 3 ng per L in the next hour. Another promising strategy simply identified patients with an initial hsTnT level of less than 5 ng per L as the ruled-out group; only 0.17% of these patients had a final diagnosis of AMI, and only 0.02% died within 30 days.3
In summary, patients with no signs of ischemia on ECG and an initially undetectable troponin T or I level using one of the new high-sensitivity assays have a very low likelihood of AMI or 30-day mortality. Similar results were found in patients with a slightly higher initial hsTnT level of up to 13 or 14 ng per L, but no or very little increase in that value over the subsequent one to two hours.
Applying the Evidence
A 54-year-old man presents to his family physician's office with chest pain that began three hours ago. The pain is nonradiating, and he feels that it may be worse with movement. His ECG shows no signs of ischemia, and you recommend that he go to the ED for further evaluation. He declines ambulance transport, and on arrival at the ED 60 minutes later, he has an initial hsTnT level of 8 ng per L. The emergency physician calls you and recommends admission for an elevated hsTnT level (the hospital uses a cutoff of 5 ng per L or greater as abnormal). Should he be admitted, or can he be safely discharged home, with evaluation the next day in your office?
Answer: The studies by Reichlin and colleagues found that an initial troponin T value less than 14 ng per L that increases by less than 4 ng per L in the next two hours (or a value less than 13 ng per L that increases by less than 3 ng per L in the next hour) effectively rules out AMI.4,5 Furthermore, those patients have an excellent prognosis, with 0% 30-day mortality in several large series.4,5 After you discuss this with the ED physician, she agrees to repeat the troponin measurement in two hours. If the level is 11 ng per L or lower, the patient will be sent home, with an appointment to see you the next day to discuss further evaluation as an outpatient.
