Am Fam Physician. 2017;95(4):248-254
Patient information: See related handout on proteinuria in children.
Author disclosure: No relevant financial affiliations.
Although proteinuria is usually benign in the form of transient or orthostatic proteinuria, persistent proteinuria may be associated with more serious renal diseases. Proteinuria may be an independent risk factor for the progression of chronic kidney disease in children. Mechanisms of proteinuria can be categorized as glomerular, tubular, secretory, or overflow. A history, a physical examination, and laboratory tests help determine the cause. Transient (functional) proteinuria is temporary. It can occur with fever, exercise, stress, or cold exposure, and it resolves when the inciting factor is removed. Orthostatic proteinuria is the most common type in children, especially in adolescent males. It is a benign condition without clinical significance. Persistent proteinuria can be glomerular or tubulointerstitial in origin. The urine dipstick test is the most widely used screening method. Although a 24-hour urine protein excretion test is usually recommended for quantitation of the amount of protein excreted in the urine, it may be impractical in children. A spot, first-morning urine test for a protein-to-creatinine or protein-to-osmolality ratio is a reliable substitute. Treatment of proteinuria should be directed at the underlying cause. Patients with active urinary sediments, hematuria, hypertension, hypocomplementemia, renal insufficiency with depressed glomerular filtration rate, or signs and symptoms suggestive of vasculitic disease may require referral to a pediatric nephrologist and a renal biopsy.
The presence of protein in urine is a common laboratory finding in children. Although proteinuria is usually benign, it can be a marker of a serious underlying renal disease or systemic disorder.1–3 When proteinuria coexists with hematuria, the likelihood of clinically significant renal disease is higher.1,2 Further, proteinuria represents an independent risk factor for the progression of nonglomerular or glomerular chronic kidney disease in children.4–9 The Chronic Kidney Disease in Children study demonstrated that persistent proteinuria with a high urine protein-to-creatinine (UPr/Cr) ratio (more than 2 in patients with nonglomerular disease and more than 0.5 in patients with glomerular disease) predicts significant chronic kidney disease progression.7 The challenge for the primary care physician is to separate benign forms of proteinuria from those with clinical significance.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| When proteinuria is present, physicians should monitor children with nonglomerular or glomerular chronic kidney disease for disease progression. | C | 4, 7, 9 |
| When a child's UPr/Cr ratio is more than 0.2 (more than 0.5 for children six to 24 months of age) or urinalysis results are abnormal, further evaluation with history, physical examination, and additional blood work is recommended to rule out significant renal disease. | C | 3 |
| A 24-hour urine protein excretion test is often difficult in children. Spot, first-morning urine testing for determining UPr-to-osmolality ratio is more useful and correlates well with the 24-hour urine collection; it should be used in children if the urine dipstick test result is 1 or more. | C | 3, 16, 19 |
| There is no evidence that early treatment with prednisone reduces the prevalence of persistent proteinuria 12 months after onset of Henoch-Schönlein purpura. | B | 11 |
| For the treatment of idiopathic nephrotic syndrome, the duration of prednisone should not be increased beyond two to three months. | A | 22 |
| In patients with renal dysfunction, treatment with an angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker appears to decrease proteinuria. | C | 5, 26, 27 |
| Patients with active urinary sediments, hematuria, hypertension, hypocomplementemia, renal insufficiency with depressed glomerular filtration rate, or signs and symptoms suggestive of vasculitic disease may require referral to a pediatric nephrologist and renal biopsy. | C | 3, 28 |
Epidemiology
Mechanisms of Proteinuria
Mechanisms of proteinuria can be categorized as glomerular, tubular, secretory, or overflow.1,2,12,13 Glomerular proteinuria is due to increased filtration of macromolecules, particularly albumin, across the glomerular capillary wall. This may be a result of increased permeability of the glomerular basement membrane because of structural defects of the membrane, loss of its negative charges, or direct damage by immune complexes.1,2,12 Glomerular proteinuria can also occur when a reduced number of functioning nephrons leads to increased diffusion of protein across the remaining glomeruli. Tubular proteinuria occurs when there is an increased excretion of normally filtered low-molecular-weight proteins because of impaired reabsorption by the proximal tubules.12 Secretory proteinuria results from oversecretion of certain proteins in the tubules, most notably the Tamm-Horsfall proteins in interstitial nephritis. Overflow proteinuria occurs when the plasma concentrations of low-molecular-weight proteins exceed the capacity of the tubules to reabsorb the filtered protein.
Etiology

| Transient (functional) proteinuria | |||
| Idiopathic | |||
| Related to a medical condition (e.g., fever, seizure) | |||
| Unrelated to a medical condition (e.g., exercise, stress, dehydration, cold exposure) | |||
| Orthostatic proteinuria | |||
| Persistent proteinuria | |||
| Glomerular | |||
| Adaptation (hyperfiltration) because of nephron loss (e.g., reflux nephropathy secondary to vesicoureteric reflux) | |||
| Alport syndrome | |||
| Collagen vascular disease or vasculitis: Henoch-Schönlein purpura, systemic lupus erythematosus | |||
| Diabetes mellitus | |||
| Glomerulopathy: congenital nephrotic syndrome, focal segmental glomerulosclerosis, immunoglobulin A nephropathy, membranoproliferative glomerulonephritis, mesangial proliferative glomerulonephritis, minimal change glomerulopathy | |||
| Infections: group A beta-hemolytic streptococcus infection, viral infection (hepatitis B and C, human immunodeficiency virus, infectious mononucleosis), other infections (malaria, syphilis) | |||
| Malignancies (lymphoma, solid tumors) | |||
| Toxin (mercury) | |||
| Tubulointerstitial | |||
| Acute tubular necrosis: aminoglycosides, amphotericin B, cisplatin, nonsteroidal anti-inflammatory drugs, radiocontrast media | |||
| Acute tubulointerstitial nephritis: nonsteroidal anti-inflammatory drugs, penicillin, cephalosporins, quinolones, sulfonamides, cimetidine (Tagamet), allopurinol | |||
| Polycystic kidney disease | |||
| Proximal renal tubular acidosis: Fanconi syndrome (global proximal tubule dysfunction), cystinosis, Lowe syndrome, galactosemia, Wilson disease | |||
| Pyelonephritis | |||
| Toxins (lead, copper, mercury) | |||
TRANSIENT PROTEINURIA
Transient (functional) proteinuria is temporary and resolves when the inciting factor remits or is removed. Transient proteinuria can occur with a medical condition (e.g., fever, seizure) or another trigger, such as exercise, stress, dehydration, or cold exposure. It can also be idiopathic.
ORTHOSTATIC PROTEINURIA
Orthostatic proteinuria is the most common cause of proteinuria in children, especially in adolescent males.1,14,15 It is a benign condition without clinical significance.1,15 The diagnosis is suggested with normal protein excretion (i.e., negative urine dipstick test result, or a UPr/Cr ratio of 0.2 or less) in a spot, first-morning urine sample after the patient has been supine for the entire night, but increased protein excretion (i.e., positive urine dipstick test result, or a UPr/Cr ratio of more than 0.2) at least four to six hours after the patient has been upright.1–3 The cause of orthostatic proteinuria is not clear; however, the anatomic compression of the left renal vein has been suggested.
PERSISTENT PROTEINURIA
Persistent proteinuria can be tubulointerstitial or more commonly glomerular in origin.1–3 Albumin and immunoglobulin G in the urine are the usual indicators for glomerular diseases. Glomerular diseases can have nephrotic and/or nephritic features, and distinguishing these features can help narrow the differential diagnosis. Nephrotic syndrome is characterized by heavy proteinuria (more than 1,000 mg per m2 per day or a UPr/Cr ratio of more than 2), edema, hypoalbuminemia (less than 2.5 g per dL [25 g per L]), and hyperlipidemia.16 Nephritic features include hematuria; hypertension; oliguria; and active urinary sediments, such as red blood cells, white blood cells, and cellular casts.
Tubulointerstitial diseases usually involve low-molecular-weight proteins. Proteinuria associated with renal tubular disorders is generally mild. Tubular proteinuria rarely presents a diagnostic dilemma because the underlying disease is usually detected before the proteinuria.17
Clinical Evaluation
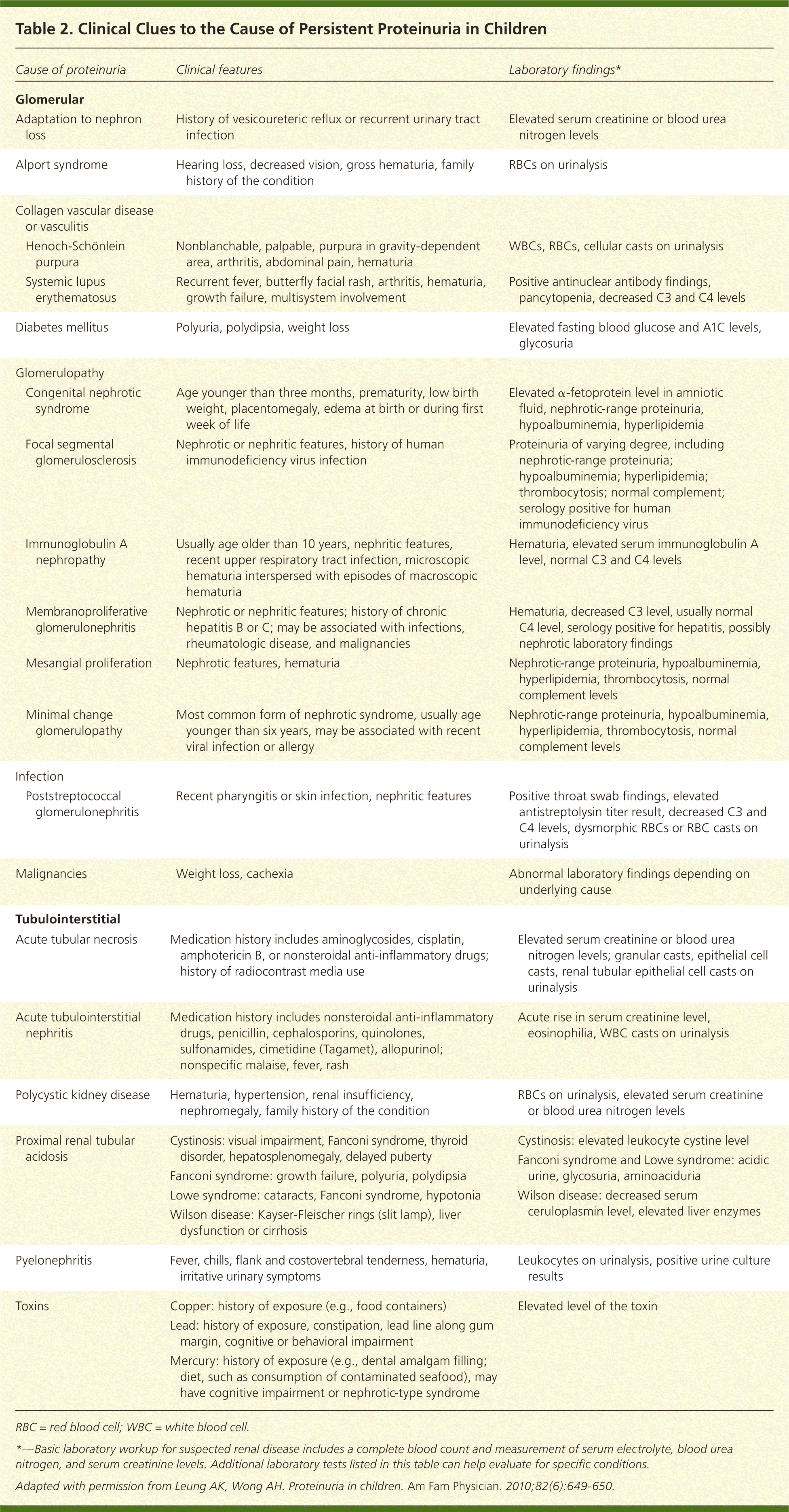
| Cause of proteinuria | Clinical features | Laboratory findings* | |
|---|---|---|---|
| Glomerular | |||
| Adaptation to nephron loss | History of vesicoureteric reflux or recurrent urinary tract infection | Elevated serum creatinine or blood urea nitrogen levels | |
| Alport syndrome | Hearing loss, decreased vision, gross hematuria, family history of the condition | RBCs on urinalysis | |
| Collagen vascular disease or vasculitis | |||
| Henoch-Schönlein purpura | Nonblanchable, palpable, purpura in gravity-dependent area, arthritis, abdominal pain, hematuria | WBCs, RBCs, cellular casts on urinalysis | |
| Systemic lupus erythematosus | Recurrent fever, butterfly facial rash, arthritis, hematuria, growth failure, multisystem involvement | Positive antinuclear antibody findings, pancytopenia, decreased C3 and C4 levels | |
| Diabetes mellitus | Polyuria, polydipsia, weight loss | Elevated fasting blood glucose and A1C levels, glycosuria | |
| Glomerulopathy | |||
| Congenital nephrotic syndrome | Age younger than three months, prematurity, low birth weight, placentomegaly, edema at birth or during first week of life | Elevated α-fetoprotein level in amniotic fluid, nephrotic-range proteinuria, hypoalbuminemia, hyperlipidemia | |
| Focal segmental glomerulosclerosis | Nephrotic or nephritic features, history of human immunodeficiency virus infection | Proteinuria of varying degree, including nephrotic-range proteinuria; hypoalbuminemia; hyperlipidemia; thrombocytosis; normal complement; serology positive for human immunodeficiency virus | |
| Immunoglobulin A nephropathy | Usually age older than 10 years, nephritic features, recent upper respiratory tract infection, microscopic hematuria interspersed with episodes of macroscopic hematuria | Hematuria, elevated serum immunoglobulin A level, normal C3 and C4 levels | |
| Membranoproliferative glomerulonephritis | Nephrotic or nephritic features; history of chronic hepatitis B or C; may be associated with infections, rheumatologic disease, and malignancies | Hematuria, decreased C3 level, usually normal C4 level, serology positive for hepatitis, possibly nephrotic laboratory findings | |
| Mesangial proliferation | Nephrotic features, hematuria | Nephrotic-range proteinuria, hypoalbuminemia, hyperlipidemia, thrombocytosis, normal complement levels | |
| Minimal change glomerulopathy | Most common form of nephrotic syndrome, usually age younger than six years, may be associated with recent viral infection or allergy | Nephrotic-range proteinuria, hypoalbuminemia, hyperlipidemia, thrombocytosis, normal complement levels | |
| Infection | |||
| Poststreptococcal glomerulonephritis | Recent pharyngitis or skin infection, nephritic features | Positive throat swab findings, elevated antistreptolysin titer result, decreased C3 and C4 levels, dysmorphic RBCs or RBC casts on urinalysis | |
| Malignancies | Weight loss, cachexia | Abnormal laboratory findings depending on underlying cause | |
| Tubulointerstitial | |||
| Acute tubular necrosis | Medication history includes aminoglycosides, cisplatin, amphotericin B, or nonsteroidal anti-inflammatory drugs; history of radiocontrast media use | Elevated serum creatinine or blood urea nitrogen levels; granular casts, epithelial cell casts, renal tubular epithelial cell casts on urinalysis | |
| Acute tubulointerstitial nephritis | Medication history includes nonsteroidal anti-inflammatory drugs, penicillin, cephalosporins, quinolones, sulfonamides, cimetidine (Tagamet), allopurinol; nonspecific malaise, fever, rash | Acute rise in serum creatinine level, eosinophilia, WBC casts on urinalysis | |
| Polycystic kidney disease | Hematuria, hypertension, renal insufficiency, nephromegaly, family history of the condition | RBCs on urinalysis, elevated serum creatinine or blood urea nitrogen levels | |
| Proximal renal tubular acidosis | Cystinosis: visual impairment, Fanconi syndrome, thyroid disorder, hepatosplenomegaly, delayed puberty | Cystinosis: elevated leukocyte cystine level | |
| Fanconi syndrome: growth failure, polyuria, polydipsia | Fanconi syndrome and Lowe syndrome: acidic urine, glycosuria, aminoaciduria | ||
| Lowe syndrome: cataracts, Fanconi syndrome, hypotonia | Wilson disease: decreased serum ceruloplasmin level, elevated liver enzymes | ||
| Wilson disease: Kayser-Fleischer rings (slit lamp), liver dysfunction or cirrhosis | |||
| Pyelonephritis | Fever, chills, flank and costovertebral tenderness, hematuria, irritative urinary symptoms | Leukocytes on urinalysis, positive urine culture results | |
| Toxins | Copper: history of exposure (e.g., food containers) | Elevated level of the toxin | |
| Lead: history of exposure, constipation, lead line along gum margin, cognitive or behavioral impairment | |||
| Mercury: history of exposure (e.g., dental amalgam filling; diet, such as consumption of contaminated seafood), may have cognitive impairment or nephrotic-type syndrome | |||
Diagnostic Evaluation
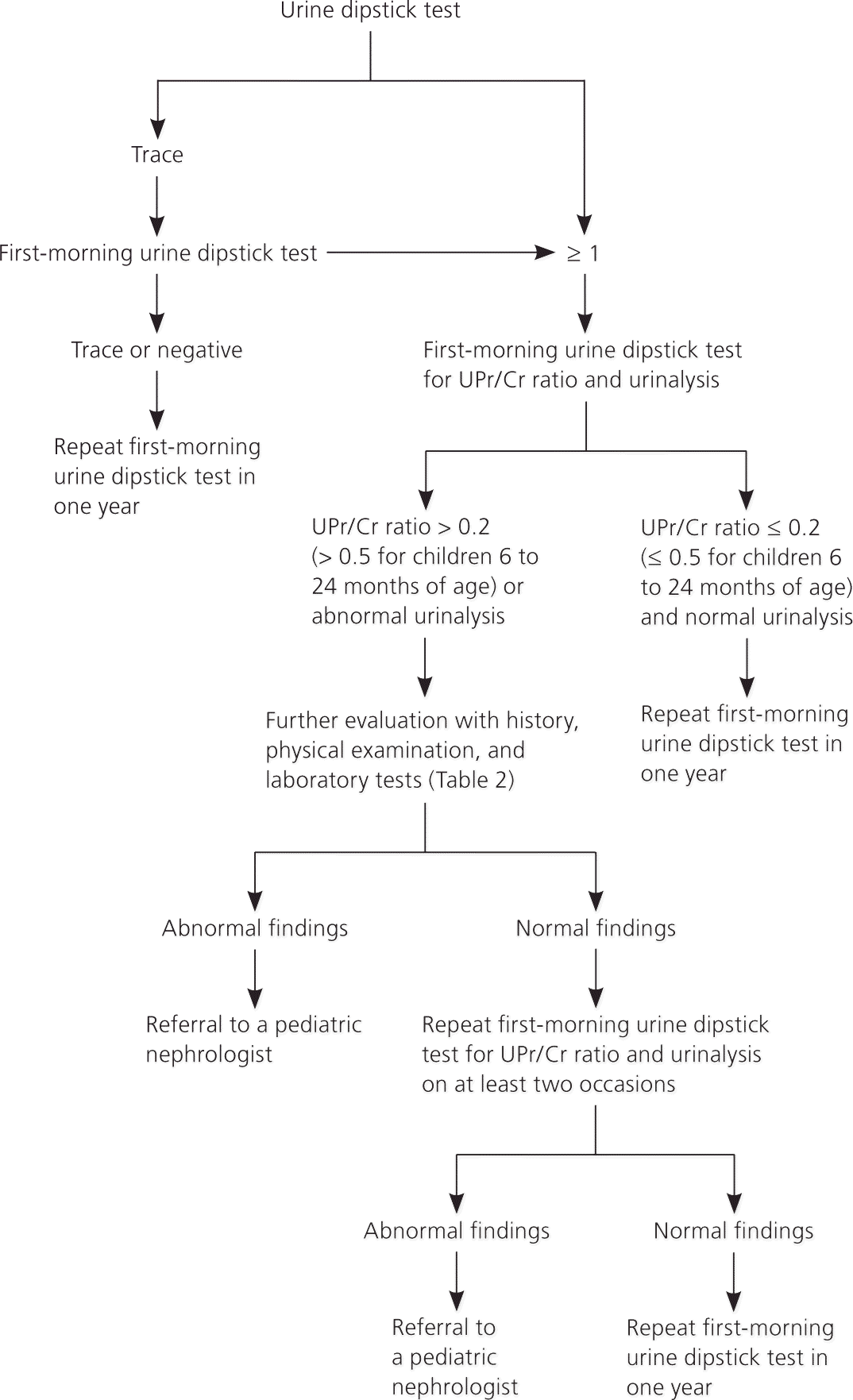
If a urine dipstick test shows trace amounts of protein, the test should be repeated with first-morning urine. If the first-morning test shows a trace or negative amount of protein, it should be repeated in one year to ensure that the proteinuria does not recur.1–3 In children with a urine dipstick test showing a result of 1 or more, a first-morning urine dipstick test for UPr/Cr ratio and a urinalysis should be performed (urine bag collection is acceptable in younger children). If the UPr/Cr ratio is 0.2 or less (0.5 or less for children six to 24 months of age) and urinalysis results are normal, transient or orthostatic proteinuria is likely.2 A repeat first-morning urine dipstick test in one year should be considered. If the UPr/Cr ratio is more than 0.2 (more than 0.5 for children six to 24 months of age) or urinalysis results are abnormal (e.g., hematuria, leukocyturia, active urinary sediments), persistent proteinuria or proteinuria of clinical significance is more likely,2 and further evaluation with history, physical examination, and additional laboratory testing is recommended to rule out significant renal disease.1–3
Measurement of Proteinuria
OFFICE TESTING
The urine dipstick test uses the tetrabromophenol blue colorimetric method and is the most widely used screening test.1,2,17 The test primarily detects albuminuria, with a specificity and sensitivity of more than 99%, but it is not sensitive for other proteins.18 The intensity of color changes from yellow to blue correlates with the amount of protein in the urine: trace (15 mg per dL), 1+ (30 mg per dL), 2+ (100 mg per dL), 3+ (300 mg per dL), and 4+ (1,000 mg per dL or more).18 A reading of 1+ or more is considered abnormal. The dipstick urine test may yield false-positive results with alkaline urine (pH greater than 8); concentrated urine (specific gravity greater than 1.030); gross hematuria; pyuria; bacteriuria; prolonged immersion of reagent strip in the urine or placement of reagent strip directly in the urine stream; and presence of phenazopyridine (Pyridium), chlorhexidine (Peridex), benzalkonium, or an iodinated radiocontrast agent in the urine.9,17 False-negative results may occur with acidic urine (pH less than 4.5), dilute urine (specific gravity less than 1.010), and presence of proteins other than albumin in the urine.9,17
The sulfosalicylic acid test detects all forms of protein and is generally used as a supplementary test when the presence of a low-molecular-weight or other protein is suspected but not detected by the dipstick urine test. In the sulfosalicylic acid method, three drops of a sulfosalicylic acid 20% solution are added to 5 mL of urine. Depending on the amount of protein precipitated, various grades of turbidity, from minimal (trace) to heavy flocculation (4+), are noted.9,17
QUANTITATIVE LABORATORY TESTING
The first-line test is a 24-hour urine protein excretion. In children, the normal amount of protein is less than 100 mg per m2 per day.3 However, this quantitative measurement is often difficult in children, particularly in those who are incontinent.3 Also, the test has an inherent time delay, is often difficult to obtain in an outpatient setting, and is subject to collection errors.
A spot, first-morning urine sample is optimal for determining UPr/Cr ratio or UPr-to-osmolality ratio because it excludes any postural effect on the protein component. The spot UPr/Cr or UPr-to-osmolality ratio is a convenient and reliable method for estimating urine protein excretion without a 24-hour urine collection.16,19–21 To estimate the total amount of protein (g per m2 per day) in the urine, the UPr/Cr ratio can be multiplied by 0.63.16
OTHER LABORATORY TESTS
A complete blood count and measurements of serum electrolytes, blood urea nitrogen, and serum creatinine should be considered if renal disease is suspected. An elevation in blood urea nitrogen or serum creatinine level suggests impaired renal function. Additional blood work should be ordered when indicated by history, physical examination, or initial laboratory results.1–3,19
IMAGING STUDIES
RENAL BIOPSY
Renal biopsy is not routinely indicated in the workup of children with proteinuria.1,2 A biopsy should be considered when proteinuria is accompanied by active urinary sediments, persistent and gross hematuria, hypertension, hypocomplementemia, renal insufficiency with depressed glomerular filtration rate (less than 60 mL per minute per 1.73 m2 for more than three months), or signs and symptoms suggestive of vasculitic disease.3
Management
The family can be reassured if the proteinuria is transient or orthostatic, and the child is asymptomatic, has no associated hematuria, and has normal blood pressure and glomerular filtration rate. However, regular follow-up is important as long as significant proteinuria persists. Although there are no formal guidelines for monitoring, a child with persistent proteinuria should initially receive a physical examination, including blood pressure measurement, urinalysis, and blood tests for creatinine and urea nitrogen levels, every six to 12 months.1,2 There is no specific limitation on diet or physical activity. Once the child is stable, follow-up can be annual.
Treatment of persistent proteinuria should be directed at the underlying cause.1–3 There is no evidence that early treatment with prednisone reduces the prevalence of persistent proteinuria 12 months after onset of Henoch-Schönlein purpura.11 The standard treatment of idiopathic nephrotic syndrome comprises four weeks of prednisone at a dosage of 60 mg per m2 per day (maximum, 80 mg per day), followed by four weeks at a dosage of 40 mg per m2 per day (maximum, 60 mg per day) taken every other day.22 There is no benefit of increasing the duration of prednisone beyond two to three months.22 If steroid therapy is ineffective or adverse effects are intolerable, second-line therapy (e.g., cyclophosphamide, chlorambucil [Leukeran], cyclosporine [Sandimmune]) may be required.23 In steroid- and cyclosporine-dependent nephrotic syndrome, biologic modifiers, such as rituximab (Rituxan), may be tried.23–25 In patients with renal dysfunction, renoprotection with an angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker may decrease proteinuria and slow progression of renal disease.5,26,27 Patients with active urinary sediments, hematuria, hypertension, hypocomplementemia, renal insufficiency with depressed glomerular filtration rate, or signs and symptoms suggestive of vasculitic disease may require referral to a pediatric nephrologist and renal biopsy.3,28
This article updates previous articles on this topic by Leung and Wong,1 and Longhman-Adham.29
Data Sources: A PubMed search was completed in Clinical Queries using the key term childhood proteinuria. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. Search date: June 1, 2016.
editor's note: This article is based in part on content from the book chapter in reference 2, which was written by Dr. Leung.