
A more recent article on COPD is available.
This is a corrected version of the article that appeared in print.
Am Fam Physician. 2017;95(7):433-441
Author disclosure: No relevant financial affiliations.
The diagnosis of chronic obstructive pulmonary disease (COPD) should be suspected in patients with risk factors (primarily a history of smoking) who report dyspnea at rest or with exertion, chronic cough with or without sputum production, or a history of wheezing. COPD may be suspected based on findings from the history and physical examination, but must be confirmed by spirometry to detect airflow obstruction. Findings that are most helpful to rule in COPD include a smoking history of more than 40 pack-years, a self-reported history of COPD, maximal laryngeal height, and age older than 45 years. The combination of three clinical variables—peak flow rate less than 350 L per minute, diminished breath sounds, and a smoking history of 30 pack-years or more—is another good clinical predictor, whereas the absence of all three of these signs essentially rules out airflow obstruction. Pharmacotherapy and smoking cessation are the mainstays of treatment, and pulmonary rehabilitation, long-term oxygen therapy, and surgery may be considered in select patients. Current guidelines recommend starting monotherapy with an inhaled bronchodilator, stepping up to combination therapy as needed, and/or adding inhaled corticosteroids as symptom severity and airflow obstruction progress.
A 53-year-old white man, Mr. J, has a history of hypertension, chronic bilateral knee pain, right knee replacement, tonsillectomy, and a 30 pack-year smoking history. He presents with a nonproductive cough that began approximately one year ago and shortness of breath for the past three months. He has no chest pain, fever, chills, night sweats, orthopnea, limb edema, or hemoptysis. Based on his smoking history and symptoms, you suspect that he has chronic obstructive pulmonary disease (COPD). This article reviews the diagnosis and management of COPD and concludes with the steps taken in the evaluation and initial treatment of Mr. J.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Screening for COPD in asymptomatic patients who are at increased risk is not recommended. | C | 3, 4, 7 |
| The diagnosis of COPD should be confirmed by a postbronchodilator FEV1/FVC ratio less than 0.7. | C | 3–5, 10 |
| Smoking cessation is recommended to reduce the rate of FEV1 decline and mortality in patients with COPD. | A | 11, 12 |
| No combination of inhaled medications has been found superior to monotherapy for initial treatment of COPD; patients should step up to combination therapy as needed. | A | 10, 15 |
| Monotherapy with a long-acting beta2 agonist or long-acting anticholinergic is recommended for symptomatic patients with COPD whose FEV1 is less than 60% of predicted. | A | 3 |
| Initial monotherapy with a long-acting beta2 agonist or long-acting anticholinergic is recommended for patients with COPD. An inhaled corticosteroid may be added as symptom severity or airflow obstruction progresses. | C | 3–5 |
| Long-term oxygen therapy is recommended for patients with COPD who have severe resting hypoxia (arterial partial pressure of oxygen 55 mm Hg or less, or oxygen saturation 88% or less). | A | 3, 25 |
| Pulmonary rehabilitation is recommended for symptomatic patients with COPD whose FEV1 is less than 50% of predicted. | A | 3, 27 |
Epidemiology
In 2013, chronic lower respiratory disease, which is comprised primarily of COPD, was the third leading cause of death in the United States.1 Its prevalence varies by state (less than 4% in Washington and Minnesota to more than 9% in Alabama and Kentucky), and an estimated 80% to 90% of cases are caused by smoking.2 This article reviews recommendations on COPD from the current literature, the Global Initiative for Chronic Obstructive Lung Disease (GOLD), the American College of Physicians (ACP), and the National Institute for Health and Care Excellence (NICE).3–5 The GOLD guidelines also were funded by the pharmaceutical industry.4
Screening
Because of the potential costs of screening and lack of clear evidence of benefit in asymptomatic persons, the ACP and GOLD guidelines do not recommend screening for COPD in patients at increased risk who do not have respiratory symptoms.3,4 The U.S. Preventive Services Task Force and AAFP also recommend against screening for COPD using spirometry.6,7
Diagnosis
COPD should be suspected in patients with risk factors who report dyspnea at rest or with exertion, chronic cough with or without sputum production, or a history of wheezing. Risk factors include age older than 35 years with significant smoking history, α1-antitrypsin deficiency, and a history of significant exposure to indoor or outdoor air pollution, occupational dusts, or chemicals. Other findings from the history and physical examination that increase the likelihood of COPD include a smoking history of more than 40 pack-years (positive likelihood ratio [LR+] = 7.3; negative likelihood ratio [LR–] = 0.5), self-reported history of COPD (LR+ = 8.3; LR– = 0.8), maximal laryngeal height of 4 cm or less (from top of thyroid cartilage to suprasternal notch; LR+ = 1.3; LR– = 0.4), and age older than 45 years regardless of smoking history (LR+ = 2.8; LR– = 0.8).8 The presence or absence of all four of these features can rule COPD in (LR+ = 220.5) or out (LR– = 0.13).8 Another good clinical predictor of COPD is the combination of three clinical variables: peak flow rate less than 350 L per minute, diminished breath sounds, and a smoking history of 30 pack-years or more.9 The presence of any one of these clinical variables predicts airflow obstruction with 98% sensitivity and 46% specificity, whereas the absence of all three essentially rules out airflow obstruction (3% false-negative rate).9
Although COPD may be suspected based on findings from the history and physical examination, the diagnosis must be confirmed by spirometry to detect airflow obstruction and its severity. Spirometry is diagnostic for COPD when the postbronchodilator forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) ratio is less than 0.7.10 The ACP, GOLD, and NICE guidelines emphasize clinical suspicion of COPD based on history and physical examination findings, with confirmation by spirometry.3–5 However, clinicians should consider that older adults or patients with comorbidities such as heart failure may also have a decreased FEV1/FVC ratio. The differential diagnosis of COPD is presented in Table 1.
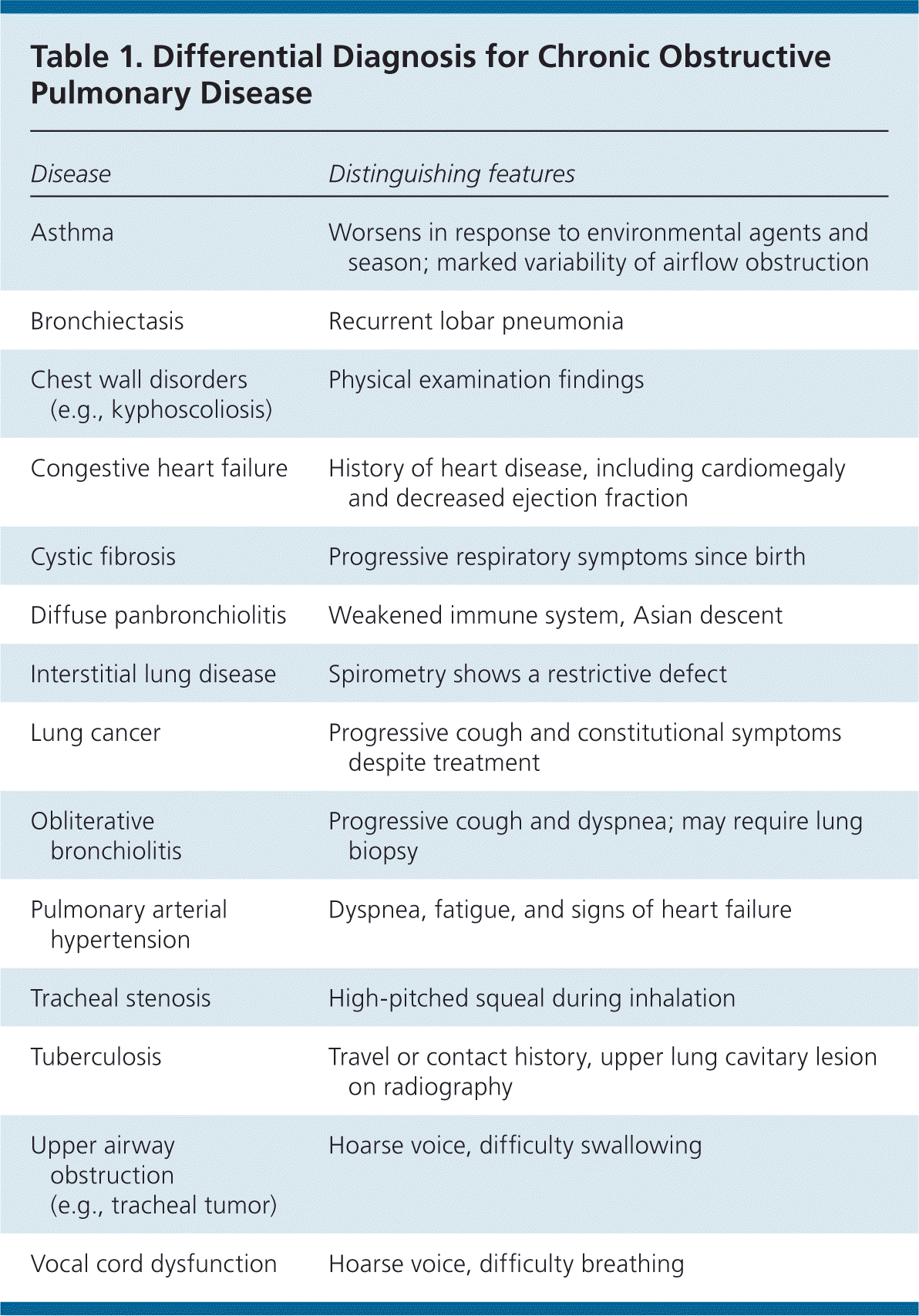
| Disease | Distinguishing features |
|---|---|
| Asthma | Worsens in response to environmental agents and season; marked variability of airflow obstruction |
| Bronchiectasis | Recurrent lobar pneumonia |
| Chest wall disorders (e.g., kyphoscoliosis) | Physical examination findings |
| Congestive heart failure | History of heart disease, including cardiomegaly and decreased ejection fraction |
| Cystic fibrosis | Progressive respiratory symptoms since birth |
| Diffuse panbronchiolitis | Weakened immune system, Asian descent |
| Interstitial lung disease | Spirometry shows a restrictive defect |
| Lung cancer | Progressive cough and constitutional symptoms despite treatment |
| Obliterative bronchiolitis | Progressive cough and dyspnea; may require lung biopsy |
| Pulmonary arterial hypertension | Dyspnea, fatigue, and signs of heart failure |
| Tracheal stenosis | High-pitched squeal during inhalation |
| Tuberculosis | Travel or contact history, upper lung cavitary lesion on radiography |
| Upper airway obstruction (e.g., tracheal tumor) | Hoarse voice, difficulty swallowing |
| Vocal cord dysfunction | Hoarse voice, difficulty breathing |
Assessment of Disease Severity
The GOLD and NICE classification systems categorize disease severity based on spirometry results (Table 23–5 ) and symptom assessment using a validated questionnaire such as the modified Medical Research Council (mMRC; http://copd.about.com/od/copdbasics/a/MMRCdyspneascale.htm) dyspnea scale or the COPD Assessment Test (CAT; http://www.catestonline.org/). These questionnaires ask about the severity of cough and activity limitations to help determine disease severity (A to D on the GOLD Combined Assessment) and effectiveness of medical interventions. The GOLD Combined Assessment uses these patient questionnaires, number of exacerbations per year, and degree of airflow obstruction to classify persons with COPD into one of four disease categories (Table 3).4 Clinicians can use these categories to help guide therapy.
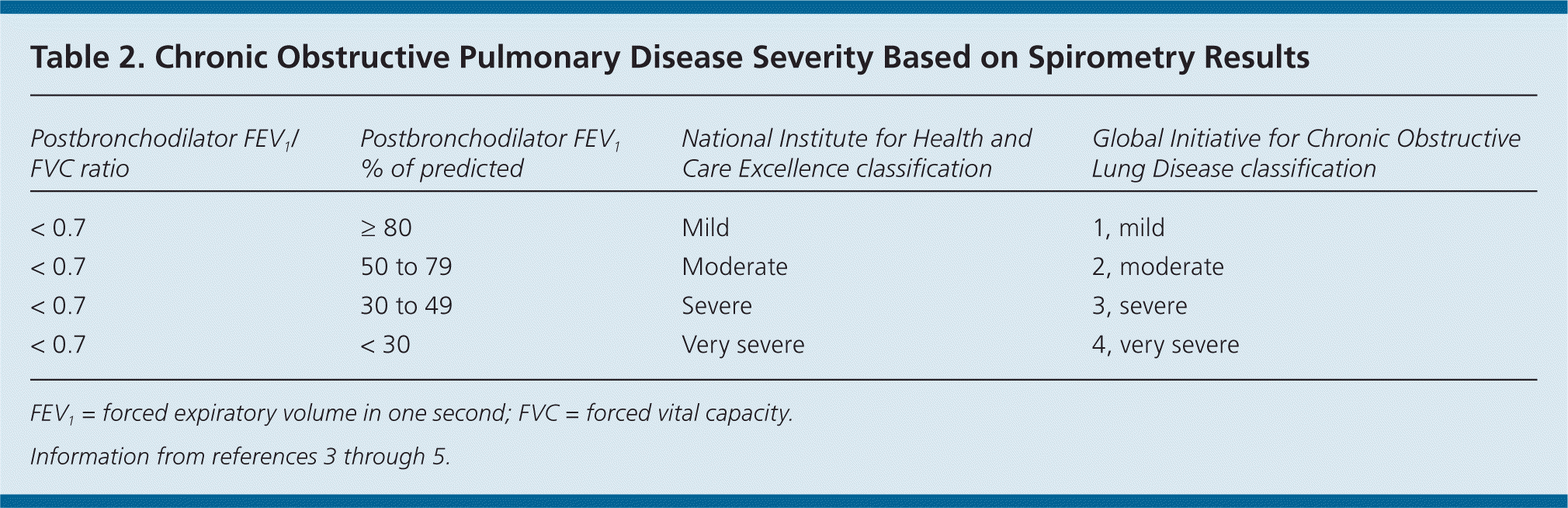
| Postbronchodilator FEV1/FVC ratio | Postbronchodilator FEV1 % of predicted | National Institute for Health and Care Excellence classification | Global Initiative for Chronic Obstructive Lung Disease classification |
|---|---|---|---|
| < 0.7 | ≥ 80 | Mild | 1, mild |
| < 0.7 | 50 to 79 | Moderate | 2, moderate |
| < 0.7 | 30 to 49 | Severe | 3, severe |
| < 0.7 | < 30 | Very severe | 4, very severe |
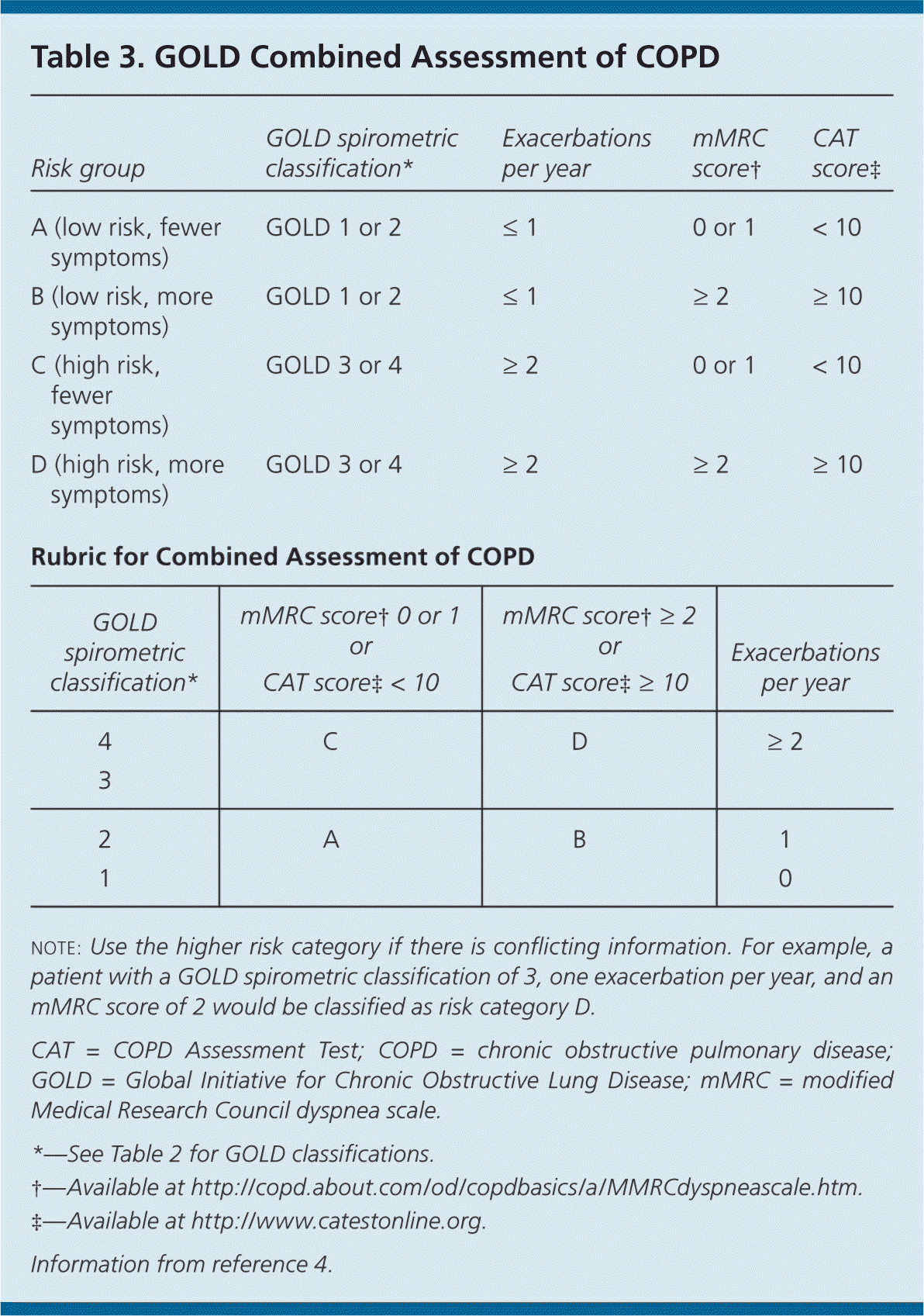
| Risk group | GOLD spirometric classification* | Exacerbations per year | mMRC score† | CAT score‡ |
|---|---|---|---|---|
| A (low risk, fewer symptoms) | GOLD 1 or 2 | ≤ 1 | 0 or 1 | < 10 |
| B (low risk, more symptoms) | GOLD 1 or 2 | ≤ 1 | ≥ 2 | ≥ 10 |
| C (high risk, fewer symptoms) | GOLD 3 or 4 | ≥ 2 | 0 or 1 | < 10 |
| D (high risk, more symptoms) | GOLD 3 or 4 | ≥ 2 | ≥ 2 | ≥ 10 |
| Rubric for Combined Assessment of COPD | ||||
| GOLD spirometric classification* | mMRC score†0 or 1 or CAT score‡< 10 | mMRC score†≥ 2 or CAT score‡≥ 10 | Exacerbations per year | |
| 4 | C | D | ≥ 2 | |
| 3 | ||||
| 2 | A | B | 1 | |
| 1 | 0 | |||
Treatment
The goals of COPD treatment are to reduce hospitalizations, reduce and prevent exacerbations, decrease dyspnea, improve quality of life, slow disease progression, and reduce mortality. The mainstays of treatment are smoking cessation, when applicable, and pharmacotherapy with inhaled bronchodilators and corticosteroids (Table 4).4 Additional therapies include oral phosphodiesterase-4 inhibitors, vaccinations, pulmonary rehabilitation, and long-term oxygen therapy in hypoxic patients.
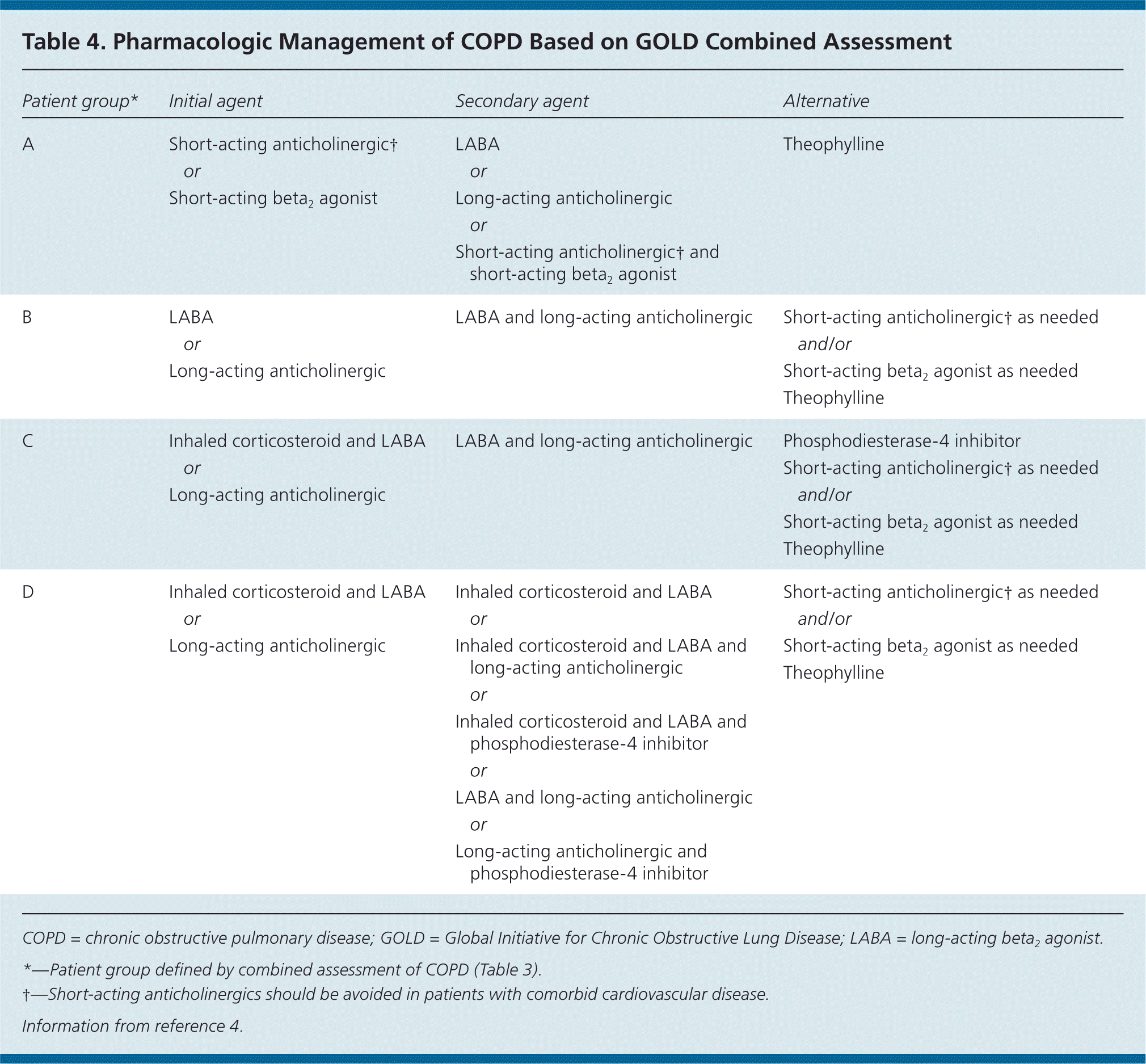
| Patient group* | Initial agent | Secondary agent | Alternative |
|---|---|---|---|
| A | Short-acting anticholinergic† | LABA | Theophylline |
| or | or | ||
| Short-acting beta2 agonist | Long-acting anticholinergic | ||
| or | |||
| Short-acting anticholinergic† and short-acting beta2 agonist | |||
| B | LABA | LABA and long-acting anticholinergic | Short-acting anticholinergic† as needed |
| or | and/or | ||
| Long-acting anticholinergic | Short-acting beta2 agonist as needed Theophylline | ||
| C | Inhaled corticosteroid and LABA | LABA and long-acting anticholinergic | Phosphodiesterase-4 inhibitor |
| or | Short-acting anticholinergic† as needed | ||
| Long-acting anticholinergic | and/or | ||
| Short-acting beta2 agonist as needed | |||
| Theophylline | |||
| D | Inhaled corticosteroid and LABA | Inhaled corticosteroid and LABA | Short-acting anticholinergic† as needed |
| or | or | and/or | |
| Long-acting anticholinergic | Inhaled corticosteroid and LABA and long-acting anticholinergic | Short-acting beta2 agonist as needed | |
| or | Theophylline | ||
| Inhaled corticosteroid and LABA and phosphodiesterase-4 inhibitor | |||
| or | |||
| LABA and long-acting anticholinergic | |||
| or | |||
| Long-acting anticholinergic and phosphodiesterase-4 inhibitor |
SMOKING CESSATION
Patients with COPD who smoke tobacco should be strongly encouraged and supported to quit. Evidence shows that smoking cessation can reduce rates of FEV1 decline and mortality.11,12 Interventions to support successful smoking cessation include pharmacotherapy with bupropion (Zyban), varenicline (Chantix), or nicotine replacement; structured support classes; and clinician's advice.12 A 2014 randomized controlled trial (RCT) examined tobacco cessation rates using combination therapy with bupropion and varenicline vs. monotherapy with varenicline.13 Cessation rates were higher with combination therapy at 12 and 26 weeks, but no difference was noted at 52 weeks.13 Although evidence is lacking to support routine screening with spirometry, one RCT showed that providing smokers older than 35 years with their lung age through spirometry in addition to patient education promoted successful smoking cessation at one year compared with a control group (13.6% and 6.4%, respectively; P = .005; number needed to treat [NNT] = 14).14
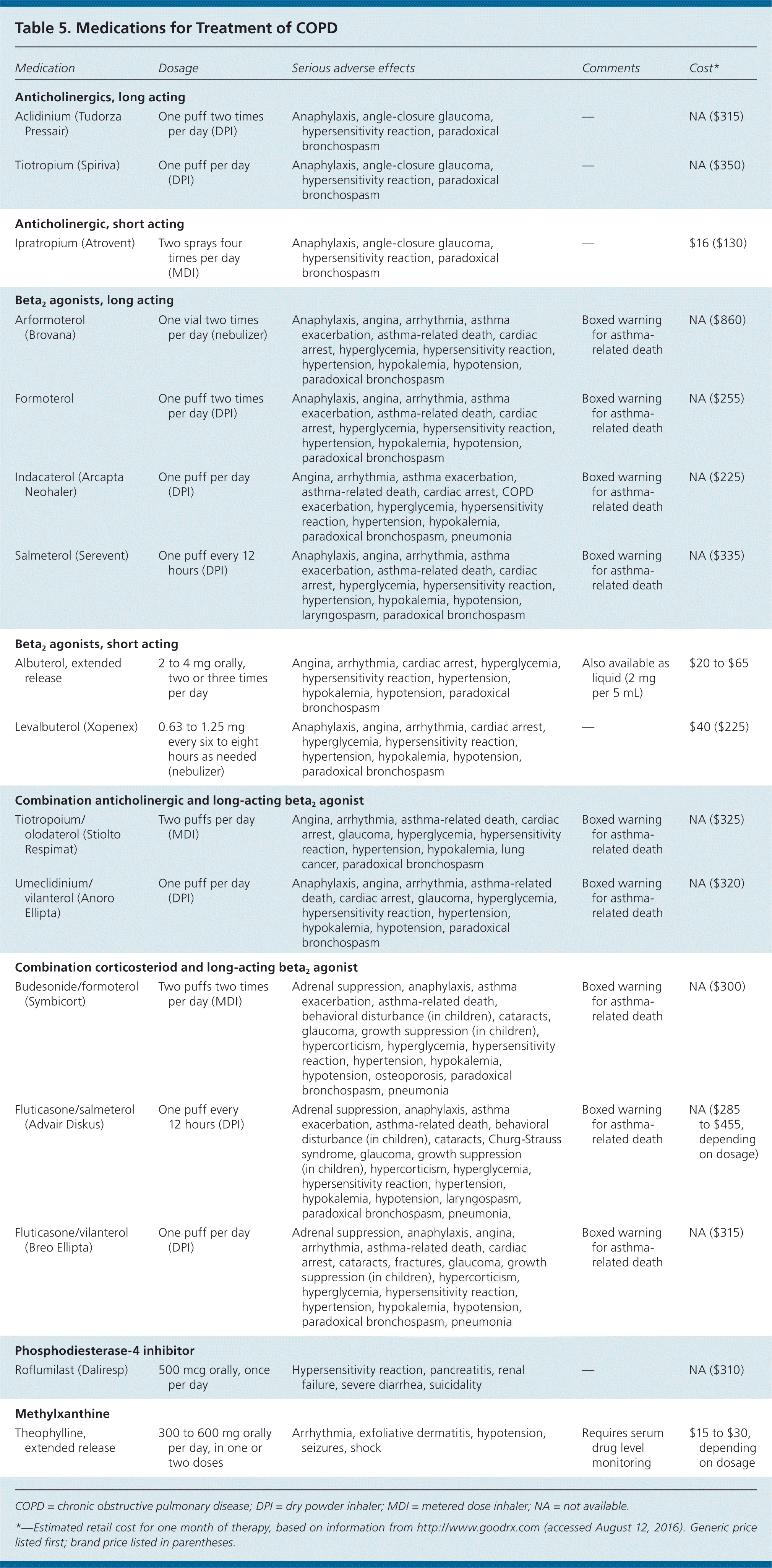
INHALED BRONCHODILATORS
There are two classes of inhaled bronchodilators: beta2 agonists and anticholinergics (Table 5). Both classes improve quality of life and decrease the annual rate of FEV1 decline (the anticholinergic tiotropium [Spiriva], 40 mL per year; long-acting beta2 agonists [LABAs], 42 mL per year) and number of exacerbations (relative risk [RR] for tiotropium = 0.84; 95% confidence interval [CI], 0.78 to 0.90; RR for LABAs = 0.87; 95% CI, 0.82 to 0.93).3,10,15,16 Studies have shown that tiotropium reduces hospitalizations for COPD exacerbations (absolute risk difference = −2%; 95% CI, –4% to –1%), and that salmeterol (Serevent) reduces annual hospitalizations by 18%.11,17 In two large clinical trials, no combination of bronchodilators has been found superior to monotherapy in decreasing COPD symptoms or preventing exacerbations.10,15 The consensus among the guidelines is to initiate COPD treatment with a single bronchodilator (LABA or anticholinergic), then step up to combination bronchodilators (LABA plus anticholinergic or LABA plus corticosteroid) if symptoms are not controlled.3–5 The ACP recommends monotherapy with a LABA or long-acting anticholinergic for symptomatic patients whose FEV1 is less than 60% of predicted.3 The choice of initial bronchodilator should be based on patient preference, adverse effects, and cost.
| Medication | Dosage | Serious adverse effects | Comments | Cost* |
|---|---|---|---|---|
| Anticholinergics, long acting | ||||
| Aclidinium (Tudorza Pressair) | One puff two times per day (DPI) | Anaphylaxis, angle-closure glaucoma, hypersensitivity reaction, paradoxical bronchospasm | — | NA ($315) |
| Tiotropium (Spiriva) | One puff per day (DPI) | Anaphylaxis, angle-closure glaucoma, hypersensitivity reaction, paradoxical bronchospasm | — | NA ($350) |
| Anticholinergic, short acting | ||||
| Ipratropium (Atrovent) | Two sprays four times per day (MDI) | Anaphylaxis, angle-closure glaucoma, hypersensitivity reaction, paradoxical bronchospasm | — | $16 ($130) |
| Beta2 agonists, long acting | ||||
| Arformoterol (Brovana) | One vial two times per day (nebulizer) | Anaphylaxis, angina, arrhythmia, asthma exacerbation, asthma-related death, cardiac arrest, hyperglycemia, hypersensitivity reaction, hypertension, hypokalemia, hypotension, paradoxical bronchospasm | Boxed warning for asthma-related death | NA ($860) |
| Formoterol | One puff two times per day (DPI) | Anaphylaxis, angina, arrhythmia, asthma exacerbation, asthma-related death, cardiac arrest, hyperglycemia, hypersensitivity reaction, hypertension, hypokalemia, hypotension, paradoxical bronchospasm | Boxed warning for asthma-related death | NA ($255) |
| Indacaterol (Arcapta Neohaler) | One puff per day (DPI) | Angina, arrhythmia, asthma exacerbation, asthma-related death, cardiac arrest, COPD exacerbation, hyperglycemia, hypersensitivity reaction, hypertension, hypokalemia, paradoxical bronchospasm, pneumonia | Boxed warning for asthma-related death | NA ($225) |
| Salmeterol (Serevent) | One puff every 12 hours (DPI) | Anaphylaxis, angina, arrhythmia, asthma exacerbation, asthma-related death, cardiac arrest, hyperglycemia, hypersensitivity reaction, hypertension, hypokalemia, hypotension, laryngospasm, paradoxical bronchospasm | Boxed warning for asthma-related death | NA ($335) |
| Beta2 agonists, short acting | ||||
| Albuterol, extended release | 2 to 4 mg orally, two or three times per day | Angina, arrhythmia, cardiac arrest, hyperglycemia, hypersensitivity reaction, hypertension, hypokalemia, hypotension, paradoxical bronchospasm | Also available as liquid (2 mg per 5 mL) | $20 to $65 |
| Levalbuterol (Xopenex) | 0.63 to 1.25 mg every six to eight hours as needed (nebulizer) | Anaphylaxis, angina, arrhythmia, cardiac arrest, hyperglycemia, hypersensitivity reaction, hypertension, hypokalemia, hypotension, paradoxical bronchospasm | — | $40 ($225) |
| Combination anticholinergic and long-acting beta2 agonist | ||||
| Tiotropoium/olodaterol (Stiolto Respimat) | Two puffs per day (MDI) | Angina, arrhythmia, asthma-related death, cardiac arrest, glaucoma, hyperglycemia, hypersensitivity reaction, hypertension, hypokalemia, lung cancer, paradoxical bronchospasm | Boxed warning for asthma-related death | NA ($325) |
| Umeclidinium/vilanterol (Anoro Ellipta) | One puff per day (DPI) | Anaphylaxis, angina, arrhythmia, asthma-related death, cardiac arrest, glaucoma, hyperglycemia, hypersensitivity reaction, hypertension, hypokalemia, hypotension, paradoxical bronchospasm | Boxed warning for asthma-related death | NA ($320) |
| Combination corticosteriod and long-acting beta2 agonist | ||||
| Budesonide/formoterol (Symbicort) | Two puffs two times per day (MDI) | Adrenal suppression, anaphylaxis, asthma exacerbation, asthma-related death, behavioral disturbance (in children), cataracts, glaucoma, growth suppression (in children), hypercorticism, hyperglycemia, hypersensitivity reaction, hypertension, hypokalemia, hypotension, osteoporosis, paradoxical bronchospasm, pneumonia | Boxed warning for asthma-related death | NA ($300) |
| Fluticasone/salmeterol (Advair Diskus) | One puff every 12 hours (DPI) | Adrenal suppression, anaphylaxis, asthma exacerbation, asthma-related death, behavioral disturbance (in children), cataracts, Churg-Strauss syndrome, glaucoma, growth suppression (in children), hypercorticism, hyperglycemia, hypersensitivity reaction, hypertension, hypokalemia, hypotension, laryngospasm, paradoxical bronchospasm, pneumonia, | Boxed warning for asthma-related death | NA ($285 to $455, depending on dosage) |
| Fluticasone/vilanterol (Breo Ellipta) | One puff per day (DPI) | Adrenal suppression, anaphylaxis, angina, arrhythmia, asthma-related death, cardiac arrest, cataracts, fractures, glaucoma, growth suppression (in children), hypercorticism, hyperglycemia, hypersensitivity reaction, hypertension, hypokalemia, hypotension, paradoxical bronchospasm, pneumonia | Boxed warning for asthma-related death | NA ($315) |
| Phosphodiesterase-4 inhibitor | ||||
| Roflumilast (Daliresp) | 500 mcg orally, once per day | Hypersensitivity reaction, pancreatitis, renal failure, severe diarrhea, suicidality | — | NA ($310) |
| Methylxanthine | ||||
| Theophylline, extended release | 300 to 600 mg orally per day, in one or two doses | Arrhythmia, exfoliative dermatitis, hypotension, seizures, shock | Requires serum drug level monitoring | $15 to $30, depending on dosage |
A large cohort study found an association between increased cardiovascular events and the use of ipratropium (Atrovent), a short-acting anticholinergic.18 Based on this finding, short-acting anticholinergics should be avoided in patients with COPD and comorbid cardiovascular disease. A meta-analysis of 110 trials showed that patients of all ages who have or are suspected of having asthma have increased asthma-related deaths, intubation, and hospitalizations when a LABA is used (6.3 events per 1,000 patient-years; 95% CI, 2.2 to 10.3).19 Based on the association between LABA use and adverse asthma-related outcomes, monotherapy with LABAs should be avoided in patients with comorbid asthma.
INHALED CORTICOSTEROIDS
The GOLD, NICE, and ACP guidelines recommend starting monotherapy with an inhaled bronchodilator.3–5 As symptom severity and airflow obstruction progress (typically FEV1 less than 50% of predicted), an inhaled corticosteroid should be added.3–5 Inhaled corticosteroids have been shown to decrease annual FEV1 decline (44 mL per year) and number of exacerbations (NNT to prevent one exacerbation in one year = 4), and to improve quality of life.3,20 There is a small increase in the risk of pneumonia with inhaled corticosteroid use, and long-term monotherapy with these agents is not recommended (odds ratio = 1.66; 95% CI, 1.38 to 2.00).21 The most commonly used inhaled corticosteroids for COPD, budesonide and fluticasone, are available in combined formulations with long-acting inhaled beta2 agonists (Table 5).
ORAL PHOSPHODIESTERASE-4 INHIBITORS
For patients in GOLD C or D classification with refractory symptoms, a selective phosphodiesterase-4 inhibitor such as roflumilast (Daliresp) should be considered (Table 5). In clinical trials, patients with moderate to severe COPD had decreased exacerbations with the use of phosphodiesterase-4 inhibitors compared with placebo (28% vs. 35%; NNT = 14.3); however, there was no improvement in quality of life or symptoms.22,23 Roflumilast is not a bronchodilator and should be used only as an adjunctive therapy.
METHYLXANTHINES AND ORAL CORTICOSTEROIDS
The adverse effects, narrow therapeutic window, and modest benefits of methylxanthines (primarily theophylline [Table 5]) limit their use in the treatment of patients with COPD, as does the need to monitor patients receiving these medications. Therefore, they are generally not recommended. Their use is supported by GOLD guidelines for patients with severe refractory symptoms; however, they should be discontinued if the patient does not respond after several weeks of therapy.4 Oral corticosteroids are not recommended for long-term treatment of COPD.3,4
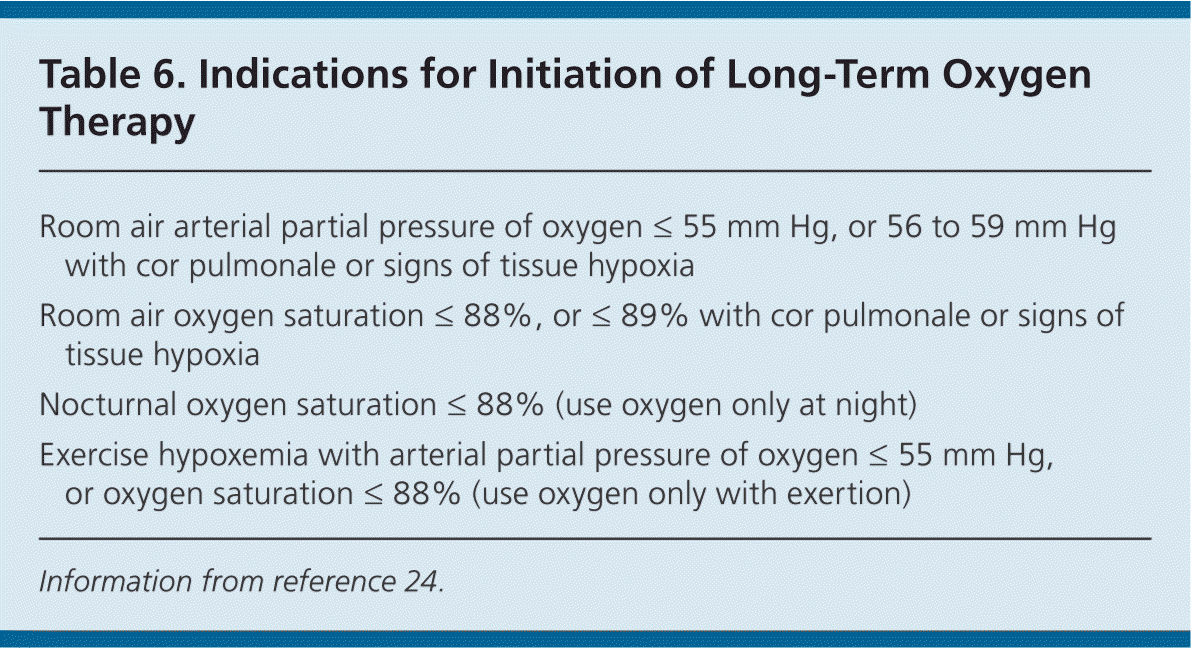
LONG-TERM OXYGEN THERAPY
Patients with moderate to severe COPD should be evaluated periodically for hypoxemia to determine the need for long-term oxygen therapy (Table 6).24 In a Cochrane review of six RCTs, oxygen therapy improved survival in select patients with COPD and severe resting hypoxemia (resting arterial partial pressure of oxygen [Pao2] less than 55 mg Hg).25 The ACP recommends long-term oxygen therapy for patients with COPD who have severe resting hypoxia (Pao2 of 55 mm Hg or less, or oxygen saturation of 88% or less).3 Hypoxemia can be evaluated with pulse oximetry after the patient has been breathing room air for 30 minutes or via measurement of arterial blood gas levels. Patients who are receiving oxygen therapy should use it for at least 15 hours per day to achieve a target oxygen saturation of 88% to 92%, or Pao2 greater than 60 mm Hg.24 Oxygen therapy can be used to decrease exertional or nocturnal dyspnea, but there is no evidence that it decreases mortality or improves health-related quality of life or daytime hypoxemia if resting Pao2 is not less than 55 mm Hg.25,26
| Room air arterial partial pressure of oxygen ≤ 55 mm Hg, or 56 to 59 mm Hg with cor pulmonale or signs of tissue hypoxia |
| Room air oxygen saturation ≤ 88%, or ≤ 89% with cor pulmonale or signs of tissue hypoxia |
| Nocturnal oxygen saturation ≤ 88% (use oxygen only at night) |
| Exercise hypoxemia with arterial partial pressure of oxygen ≤ 55 mm Hg, or oxygen saturation ≤ 88% (use oxygen only with exertion) |
PULMONARY REHABILITATION
Pulmonary rehabilitation consists of structured programs with multidisciplinary health care teams to provide exercise training, education, nutritional counseling, and behavioral modification. A meta-analysis of 20 RCTs showed that pulmonary rehabilitation reduced dyspnea and increased exercise ability and health-related quality of life, but required at least six months to achieve benefits.27 Patients most likely to benefit are those with severe COPD who have impaired quality of life and dyspnea that limits activity; however, patients with moderate COPD may also benefit.3,4,28 Clinicians should prescribe pulmonary rehabilitation for symptomatic patients whose FEV1 is less than 50% of predicted. Pulmonary rehabilitation should be considered for symptomatic or exercise-limited patients whose FEV1 is greater than 50% of predicted.
SURGERY
Although it is expensive and associated with high mortality, lung volume reduction surgery and lung transplantation may be appropriate in select patients, such as those with upper lobe–predominant emphysema or low exercise capacity before treatment.4,29 In select patients with severe upper lobe–predominant emphysema and low postrehabilitation exercise capacity, lung volume reduction surgery was associated with improved survival (54% vs. 40% in patients receiving medical therapy only over five years).4
VACCINATIONS
The Centers for Disease Control and Prevention's Advisory Committee on Immunization Practices recommends influenza vaccination for persons with COPD, and pneumococcal vaccination for persons 19 to 64 years of age who smoke or have COPD.30 The influenza vaccine should be given yearly and can reduce COPD exacerbations.1,24,31,32 One dose of 23-valent pneumococcal polysaccharide vaccine (PPSV23; Pneumovax) is recommended for persons 19 to 64 years of age who smoke or have COPD; after 65 years of age, both pneumococcal vaccines (PPSV23 and 13-valent pneumococcal conjugate vaccine [Prevnar]) should be administered one year apart.30
ANTITUSSIVES, MUCOLYTICS, AND ANTIBIOTICS
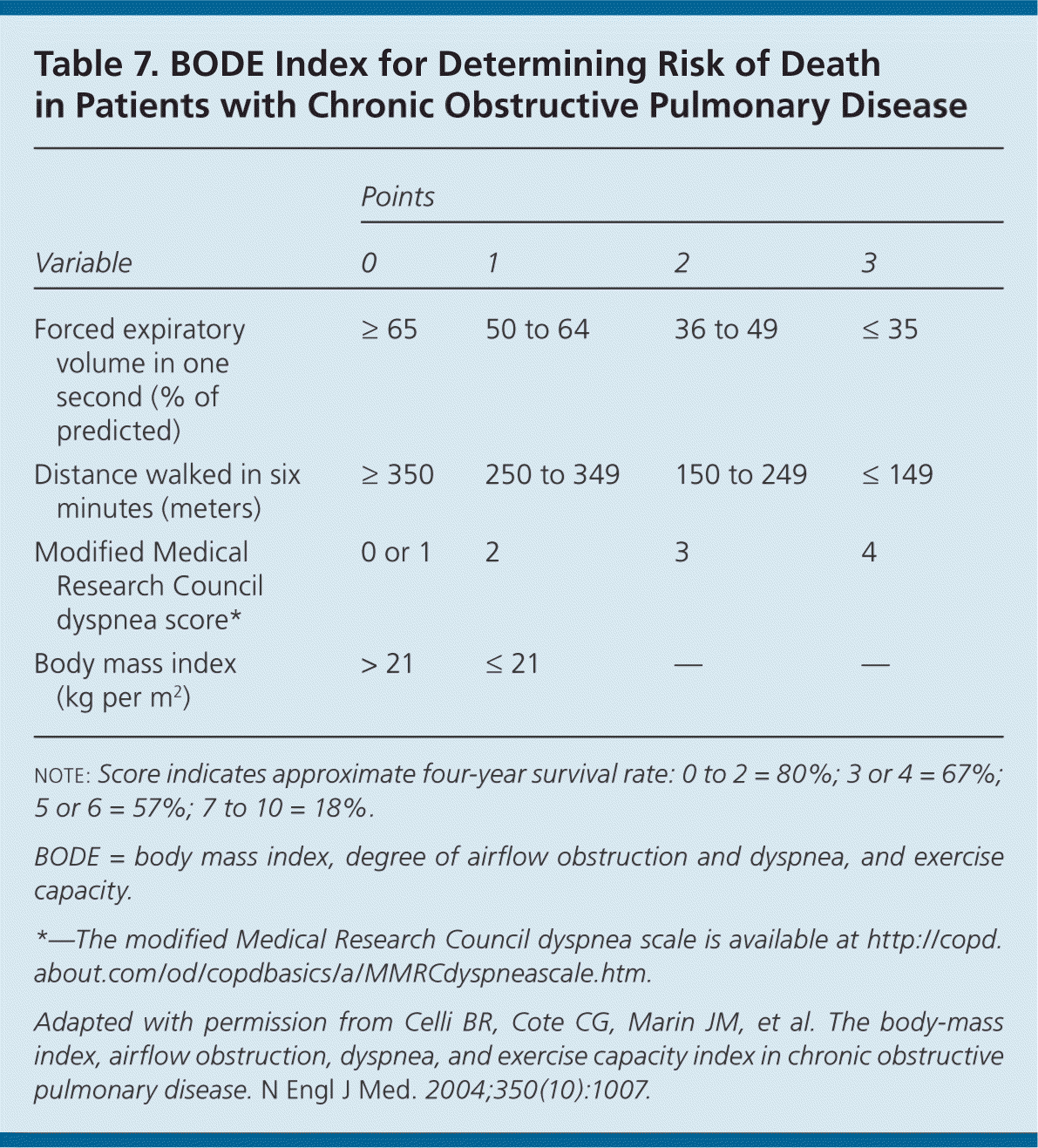
Prognosis
| Points | ||||
|---|---|---|---|---|
| Variable | 0 | 1 | 2 | 3 |
| Forced expiratory volume in one second (% of predicted) | ≥ 65 | 50 to 64 | 36 to 49 | ≤ 35 |
| Distance walked in six minutes (meters) | ≥ 350 | 250 to 349 | 150 to 249 | ≤ 149 |
| Modified Medical Research Council dyspnea score* | 0 or 1 | 2 | 3 | 4 |
| Body mass index (kg per m2) | > 21 | ≤ 21 | — | — |
Case Study Resolution
Spirometry is ordered for Mr. J to evaluate for COPD based on his symptoms and history. He is diagnosed with COPD and has a postbronchodilator FEV1 of 63% of predicted, which puts him in the moderate disease category. He scores 11 on the CAT questionnaire and reports no exacerbations, putting him in the GOLD B category. Based on these findings, the ACP recommendations (eTable A) and the GOLD guidelines, an inhaled bronchodilator is initiated. Because Mr. J is concerned about the cost of his medication, salmeterol dry powder inhaler is chosen as the initial agent. Mr. J is strongly encouraged to stop smoking and is offered tobacco cessation support classes and a nicotine-replacement agent. He is told to return for reevaluation in two weeks to ensure that his symptoms are controlled, to discuss any concerns, to confirm that he is using his medication appropriately, and to report on his progress with smoking cessation.

| Spirometry should be obtained to diagnose airflow obstruction in patients with respiratory symptoms. (Grade: strong.) Spirometry should not be used to screen for airflow obstruction in patients without respiratory symptoms. (Grade: strong.) |
| Inhaled bronchodilators may be used for patients with stable COPD who have respiratory symptoms and FEV1 of 60% to 80% of predicted. (Grade: weak.) |
| Inhaled bronchodilators are recommended for patients with stable COPD who have respiratory symptoms and FEV1 < 60% of predicted. (Grade: strong.) |
| Monotherapy with a LABA or long-acting anticholinergic is recommended for symptomatic patients with COPD who have FEV1 < 60% of predicted. (Grade: strong.) The choice of specific therapy should be based on patient preference, cost, and adverse effect profile. |
| Combination inhaled therapies (LABA, long-acting anticholinergic, or inhaled corticosteroid) may be used for symptomatic patients with stable COPD who have FEV1 < 60% of predicted. (Grade: weak.) |
| Pulmonary rehabilitation is recommended for symptomatic patients with FEV1 < 50% of predicted. (Grade: strong.) Pulmonary rehabilitation may be considered for symptomatic or exercise-limited patients with FEV1 > 50% of predicted. (Grade: weak.) |
| Continuous oxygen therapy is recommended for patients with COPD who have severe resting hypoxia (arterial partial pressure of oxygen ≤ 55 mm Hg, oroxygen saturation ≤ 88%). (Grade: strong.) |
This article updates previous articles on this topic by Lee, et al.34 ; Stephens and Yew35 ; and Grimes, et al.36
Data Sources: A PubMed search was completed in Clinical Queries using the key terms COPD, chronic bronchitis, diagnosis, and treatment. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. We also searched the Cochrane database, Essential Evidence Plus, Ovid, the National Guideline Clearinghouse database, and DynaMed. Search dates: April 2015 and July 2016.
The views expressed in this article are those of the authors and do not necessarily reflect the official policy of the Department of the Navy, Department of Defense, or the U.S. government.
