
A more recent article on testerone replacement therapy for male hypogonadism is available.
This is a corrected version of the article that appeared in print.
Am Fam Physician. 2017;96(7):441-449
Related editorial: Treating Aging with Testosterone.
Author disclosure: No relevant financial affiliations.
Testosterone therapy is increasingly common in the United States, and many of these prescriptions are written by primary care physicians. There is conflicting evidence on the benefit of male testosterone therapy for age-related declines in testosterone. Physicians should not measure testosterone levels unless a patient has signs and symptoms of hypogonadism, such as loss of body hair, sexual dysfunction, hot flashes, or gynecomastia. Depressed mood, fatigue, decreased strength, and a decreased sense of vitality are less specific to male hypogonadism. Testosterone therapy should be initiated only after two morning total serum testosterone measurements show decreased levels, and all patients should be counseled on the potential risks and benefits before starting therapy. Potential benefits of therapy include increased libido, improved sexual function, improved mood and well-being, and increased muscle mass and bone density; however, there is little or mixed evidence confirming clinically significant benefits. The U.S. Food and Drug Administration warns that testosterone therapy may increase the risk of cardiovascular complications. Other possible risks include rising prostate-specific antigen levels, worsening lower urinary tract symptoms, polycythemia, and increased risk of venous thromboembolism. Patients receiving testosterone therapy should be monitored to ensure testosterone levels rise appropriately, clinical improvement occurs, and no complications develop. Testosterone therapy may also be used to treat hypoactive sexual desire disorder in postmenopausal women and to produce physical male sex characteristics in female-to-male transgender patients.
The use of testosterone therapy is increasingly common in the United States, with an estimated 2.3 million American men receiving the therapy in 2013.1 More than one-half of testosterone prescriptions are written by primary care physicians.2 Most of these prescriptions are for middle-aged and older men with age-related declines in testosterone,1 despite inconclusive data on testosterone therapy's safety and effectiveness for this indication.
WHAT IS NEW ON THIS TOPIC: TESTOSTERONE THERAPY
Male hypogonadism should be diagnosed only if there are signs or symptoms of hypogonadism and total serum testosterone levels are low on at least two occasions.
The U.S. Food and Drug Administration clarified in 2015 that prescribing testosterone for low testosterone levels due to aging constitutes off-label use.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Testosterone therapy should be considered for men with low testosterone levels and clinical symptoms of hypogonadism, particularly sexual dysfunction. | B | 10, 12–23, 25–37 |
| Before starting treatment, male hypogonadism should be documented with low morning testosterone levels on two occasions. | C | 9 |
| Men considering testosterone therapy should be counseled about the uncertainty of the long-term safety of testosterone, including possible cardiovascular harms, and patients and physicians should engage in shared decision making, weighing the risks and benefits of therapy. | C | 9, 11, 38 |
| Men receiving testosterone therapy should be monitored regularly for adverse effects and treatment effectiveness, including testosterone measurements, complete blood count to measure hematocrit, and prostate-specific antigen testing. | C | 9, 11 |
| Testosterone therapy may be considered for treatment of postmenopausal women with hypoactive sexual desire disorder. | B | 65 |
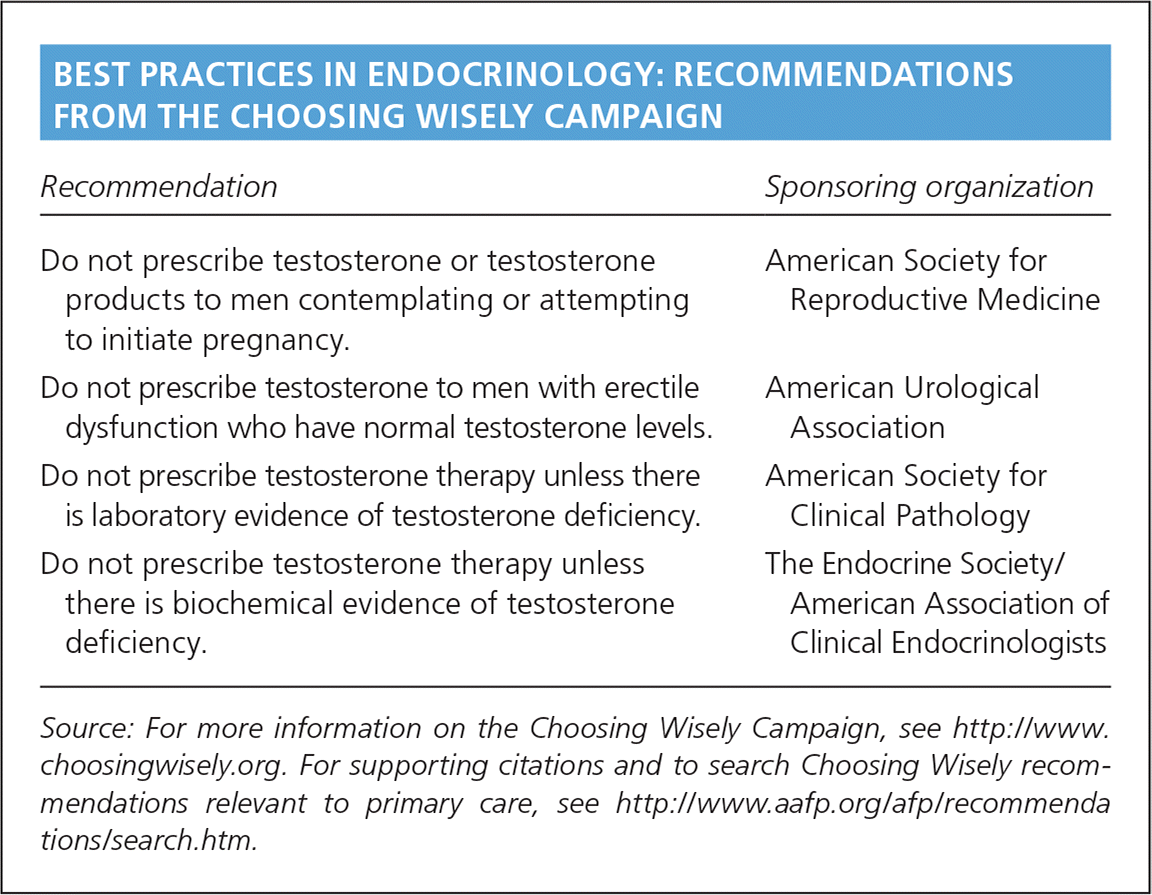
| Recommendation | Sponsoring organization |
|---|---|
| Do not prescribe testosterone or testosterone products to men contemplating or attempting to initiate pregnancy. | American Society for Reproductive Medicine |
| Do not prescribe testosterone to men with erectile dysfunction who have normal testosterone levels. | American Urological Association |
| Do not prescribe testosterone therapy unless there is laboratory evidence of testosterone deficiency. | American Society for Clinical Pathology |
| Do not prescribe testosterone therapy unless there is biochemical evidence of testosterone deficiency. | The Endocrine Society/American Association of Clinical Endocrinologists |
Physiology of Testosterone and Causes of Hypogonadism in Males
Testosterone is produced by Leydig cells in the testes, in response to luteinizing hormone produced by the pituitary gland. Decreased production of testosterone by testes in men is categorized as hypogonadism, which is classified as primary, secondary, or mixed. Primary hypogonadism is the failure of the testes to produce sufficient testosterone, whereas secondary hypogonadism is caused by decreased production of luteinizing hormone.3 Hypogonadism may also be classified by timing of onset (i.e., pre- or postpubertal). Table 1 lists the most common causes of hypogonadism.4,5
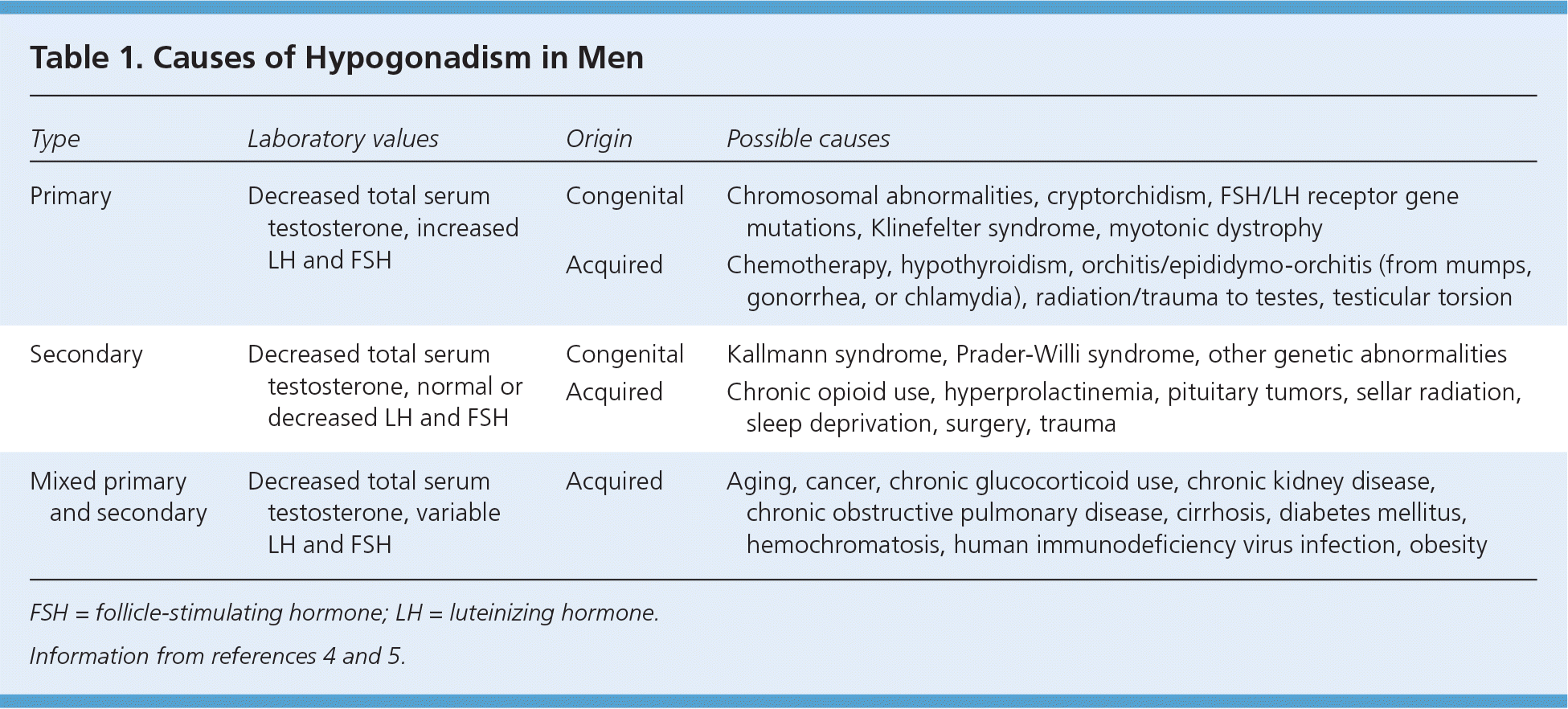
| Type | Laboratory values | Origin | Possible causes |
|---|---|---|---|
| Primary | Decreased total serum testosterone, increased LH and FSH | Congenital | Chromosomal abnormalities, cryptorchidism, FSH/LH receptor gene mutations, Klinefelter syndrome, myotonic dystrophy |
| Acquired | Chemotherapy, hypothyroidism, orchitis/epididymo-orchitis (from mumps, gonorrhea, or chlamydia), radiation/trauma to testes, testicular torsion | ||
| Secondary | Decreased total serum testosterone, normal or decreased LH and FSH | Congenital | Kallmann syndrome, Prader-Willi syndrome, other genetic abnormalities |
| Acquired | Chronic opioid use, hyperprolactinemia, pituitary tumors, sellar radiation, sleep deprivation, surgery, trauma | ||
| Mixed primary and secondary | Decreased total serum testosterone, variable LH and FSH | Acquired | Aging, cancer, chronic glucocorticoid use, chronic kidney disease, chronic obstructive pulmonary disease, cirrhosis, diabetes mellitus, hemochromatosis, human immunodeficiency virus infection, obesity |
Testosterone levels begin to decline around 40 years of age. By 80 years of age, more than 50% of men will have testosterone levels in the low range (using a reference range defined by nonobese, healthy men younger than 40 years).3 Several common medical conditions (e.g., obesity, type 2 diabetes mellitus, chronic obstructive pulmonary disease, chronic kidney disease, human immunodeficiency virus infection) and opioid dependence have been associated with low testosterone levels.6,7
Diagnosis of Male Hypogonadism
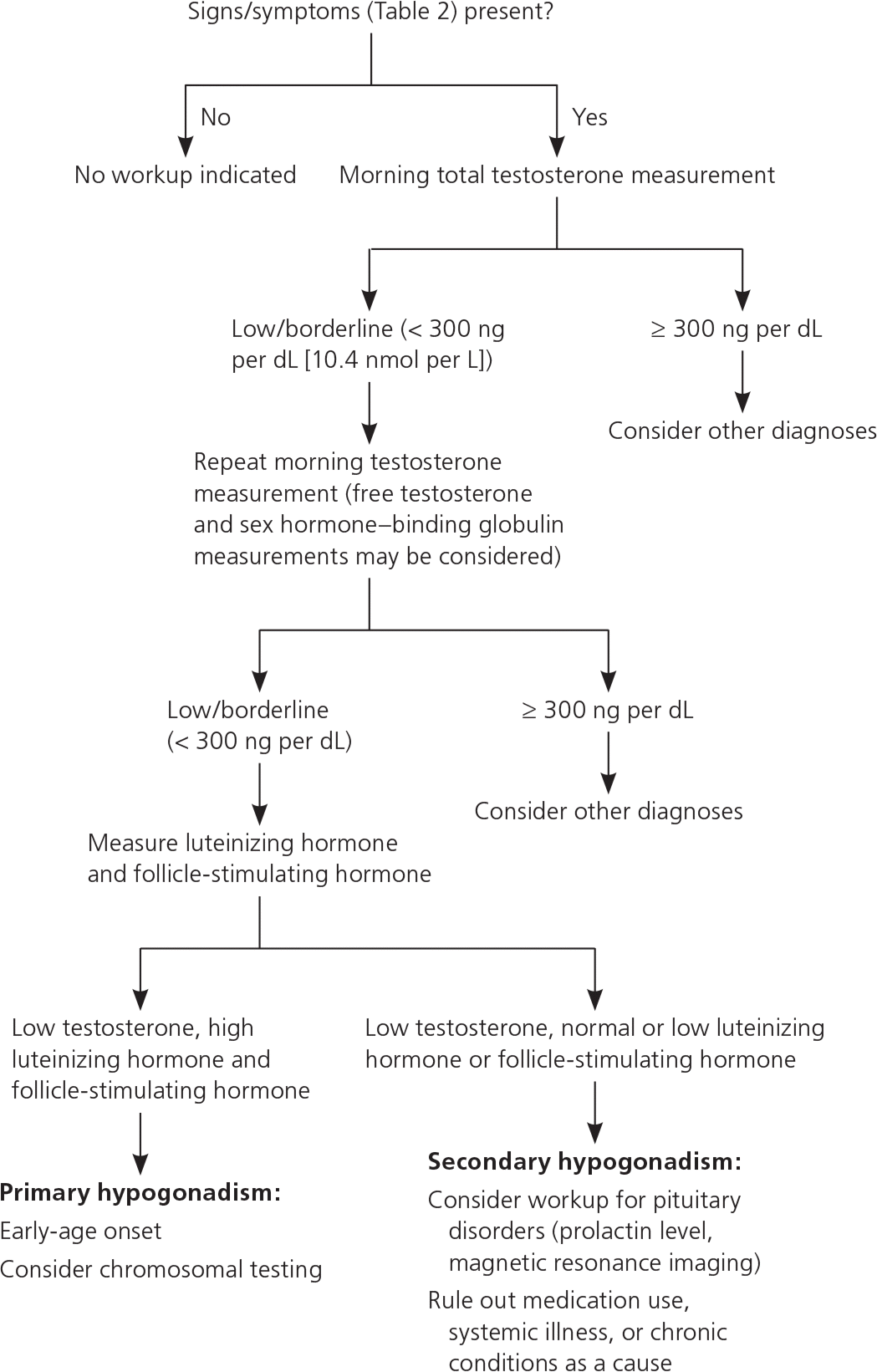
According to guidelines from the Endocrine Society, male hypogonadism should be diagnosed only if there are signs or symptoms of hypogonadism (Table 23,8,9 ) and total serum testosterone levels are low on at least two occasions.9 When diagnosing hypogonadism, physicians should not rely solely on questionnaires such as Androgen Deficiency in Aging Males or Aging Males' Symptoms because of their low sensitivity and specific-ity.9,10 Two editorials published previously in American Family Physician discuss the pros and cons of screening for testosterone deficiency (https://www.aafp.org/afp/2015/0215/p220.html and https://www.aafp.org/afp/2015/0215/p226.html).

| Anemia (normocytic, normochromic)* |
| Breast discomfort, gynecomastia |
| Depressed mood* |
| Diminished bone density, low-trauma fractures |
| Diminished energy, sense of vitality, or sense of well-being* |
| Diminished muscle mass and strength* |
| Diminished physical or work performance* |
| Hot flashes, sweats |
| Impaired cognition* |
| Incomplete or delayed sexual development (in cases of prepubertal onset) |
| Increased body fat, body mass index* |
| Increased fatigue* |
| Infertility |
| Loss of body hair |
| Sexual symptoms (decreased libido, decreased spontaneous erection) |
| Very small testes |
Because of circadian variations in testosterone levels, serum testosterone measurement should occur in the morning, or within two hours of awakening in shift workers (Figure 19 ). Although there is no universal laboratory definition of hypogonadism, in most laboratory reference ranges, the lower limit of normal is between 250 and 350 ng per dL (8.7 to 12.2 nmol per L). In men with borderline total testosterone levels, measurement of free testosterone and sex hormone–binding globulin levels should be considered, especially in the presence of conditions that affect sex hormone–binding globulin levels (most commonly, aging, obesity, and diabetes). If low testosterone is confirmed, luteinizing hormone and follicle-stimulating hormone levels should be measured to categorize the deficiency as primary or secondary.9,11 A prolactin measurement should be considered to rule out pituitary adenoma, especially if luteinizing hormone and follicle-stimulating hormone levels are low.
Benefits of Testosterone Replacement Therapy
LIBIDO AND ERECTILE FUNCTION
A common indication for testosterone therapy is treatment of decreased sexual desire or erectile dysfunction. A systematic review found 23 randomized trials of testosterone therapy's effects on libido; 13 trials showed some benefit, eight showed no benefit, and two had mixed results.12
Although evidence regarding erectile dysfunction is mixed, young men with hypogonadism and erectile dysfunction appear to benefit from testosterone therapy.13 Some studies have shown improvement in erectile dysfunction in older men and men with comorbid conditions,14,15 whereas others have not.12,16,17 Moreover, even in positive studies, the effect of testosterone has been smaller than the effect traditionally reported with phosphodiesterase-5 inhibitors,14 suggesting that testosterone should not be first-line treatment for erectile dysfunction. There is some evidence supporting the use of testosterone therapy as second-line therapy in men with low testosterone when phosphodiesterase-5 inhibitors are ineffective.18,19 There is no evidence that testosterone improves erectile function in men with normal testosterone levels. As part of the Choosing Wisely campaign, the American Urological Association says physicians should not prescribe testosterone therapy for men with erectile dysfunction and normal testosterone levels.20
BONE DENSITY, BODY COMPOSITION, AND MUSCLE STRENGTH
Low testosterone levels (less than 200 ng per dL [7.0 nmol per L]) are associated with decreased bone density and unfavorable body composition changes.21 Testosterone therapy increases bone density at the lumbar spine but not at the hip in middle-aged men with testosterone deficiency.22 In older men, testosterone therapy increases bone density in the spine and hip.23,24 There is no evidence that testosterone therapy leads to decreased fractures or falls. Testosterone therapy consistently increases lean mass and decreases fat mass,25–27 but the effect sizes are small and studies have generally failed to demonstrate improvement in strength or physical function.22,23,25,26
DEPRESSION, MOOD, COGNITION, WELL-BEING, VITALITY
The few studies of testosterone therapy for depressed mood had mixed results.28–31 Testosterone therapy does not improve cognitive function in men with or without preexisting cognitive impairment.32–34 There is also mixed evidence for prescribing testosterone to improve vitality, general quality of life, and male “symptoms of aging,” with some studies demonstrating improvement with therapy,35,36 and other studies finding no change.10,37
Testosterone and Cardiovascular Health
In a 2015 advisory, the U.S. Food and Drug Administration (FDA) warned that testosterone use is possibly associated with increased cardiovascular risk and advised physicians to discuss this risk with patients before initiating testosterone therapy.38 This warning came after two observational studies39,40 and a meta-analysis of randomized controlled trials41 showed an increased cardiovascular risk, and the Testosterone in Older Men with Mobility Limitation (TOM) randomized controlled trial was stopped early because of concerns about a higher incidence of cardiovascular adverse events in the testosterone treatment group.42 However, other meta-analyses did not find an increased cardiovascular risk,43,44 and several other observational studies have appeared to demonstrate decreased cardiovascular risk with testosterone therapy.45–48 Additionally, one of the observational studies that showed increased risk was criticized for its statistical analyses,40 and many of the adverse events leading to the early stoppage of the TOM trial were of questionable clinical significance. Although the findings of the TOM trial are concerning, this study enrolled a high-risk population, and its findings may not be generalizable to most men being considered for testosterone therapy.
Proponents of testosterone therapy point to a large number of observational studies consistently finding higher cardiovascular morbidity and mortality in men with low baseline testosterone levels and suggest that treating low testosterone should lead to decreased risk,49 although it is unclear whether low testosterone was a cause of the increased cardiovascular risk or merely a marker of poor overall health. No randomized controlled trial has demonstrated decreased cardiovascular events or mortality with testosterone therapy.
A recent systematic review found some evidence of benefit in congestive heart failure and increased time to ST segment depression in exercise testing. The review found inconsistent effects of testosterone therapy on lipids and no beneficial effect on reported angina.12
The effects of testosterone therapy on cardiovascular health remain unclear. The FDA has mandated that testosterone product manufacturers conduct a large-scale randomized controlled trial specifically to determine cardiovascular risk,38 but results of any such trial would not be available for years. In the meantime, physicians must counsel patients that the cardiovascular risks and benefits of testosterone therapy are uncertain and should engage in shared decision making.9,11,38
Risks of Testosterone Therapy and Contraindications

| Absolute contraindications |
| Breast cancer |
| Polycythemia (hematocrit > 54%) |
| Prostate cancer |
| Prostate-specific antigen > 4 ng per mL (4 mcg per L) or presence of nodules/induration on digital rectal examination (referral to a urologist is required before considering testosterone therapy) |
| Relative contraindications |
| Baseline hematocrit > 50%* |
| Desire for fertility (testosterone therapy suppresses spermatogenesis) |
| Severe lower urinary tract symptoms |
| Uncontrolled congestive heart failure |
| Untreated obstructive sleep apnea |
PROSTATE CANCER AND LOWER URINARY TRACT SYMPTOMS
Because prostate cancer can be stimulated by testosterone, testosterone therapy is contraindicated in patients with known or suspected prostate cancer. There has long been concern that testosterone therapy may increase the risk of developing prostate cancer and increase the symptoms of benign prostatic hyperplasia. However, several meta-analyses of randomized controlled trials have not shown an increased incidence of prostate cancer.50–52 Use of testosterone therapy in men with hypogonadism and previously treated (and presumed cured) prostate cancer is controversial, with little data to guide treatment decisions in this group.53
Use of supplemental testosterone has been shown to cause a small increase in prostate-specific antigen (PSA) levels,52 but the significance of this increase is questionable. There also does not appear to be a significant increase in lower urinary tract symptoms with testosterone therapy, although most studies have excluded men with severe lower urinary tract symptoms at baseline.54
HEMATOLOGIC CONDITIONS
Testosterone stimulates erythropoiesis, and testosterone therapy (in particular the intramuscular esters) is associated with an increased risk of polycythemia.50 Preexisting polycythemia (hematocrit of more than 54%) is an absolute contraindication to starting testosterone therapy. Development of polycythemia during treatment should lead to cessation of therapy, lowering of the dose, or switching to a lower-risk formulation to avoid increased risk of myocardial infarction, stroke, and venous thromboembolism. Testosterone therapy has been shown to increase hemoglobin levels and correct anemia in a significant portion of older men with anemia of otherwise unknown etiology. Testosterone measurement should be considered in older men with unexplained anemia.55
VENOUS THROMBOEMBOLISM
Based on postmarket reports, in 2014 the FDA required manufacturers of testosterone products to add a warning to the drug label about the risk of venous thromboem-bolism.56 Subsequently, a large case-control study and another large retrospective cohort study found no evidence of increased venous thromboembolism risk.57,58
Testosterone Replacement for Male Hypogonadism
FDA-INDICATED USES
As part of its 2015 advisory on cardiovascular risk, the FDA also issued a statement clarifying that testosterone therapy is approved specifically for men with low testosterone levels caused by disorders of the testicles, pituitary gland, or brain that cause hypogonadism (i.e., genetic disorders, damage from chemotherapy or infection, or pituitary tumors) and not for men with age-related low testosterone.38 Physicians should be aware that prescribing testosterone for low testosterone levels due to aging constitutes off-label use.
FORMULATIONS AND PRECAUTIONS
Many testosterone formulations are available (Table 459,60 ), and no formulation has superior clinical effects. The selection of formulation requires discussion about administration route, adverse effects, and cost. Testosterone preparations are FDA Schedule III controlled substances that are subject to diversion and misuse. Completion of a controlled substance contract should be considered before prescribing.
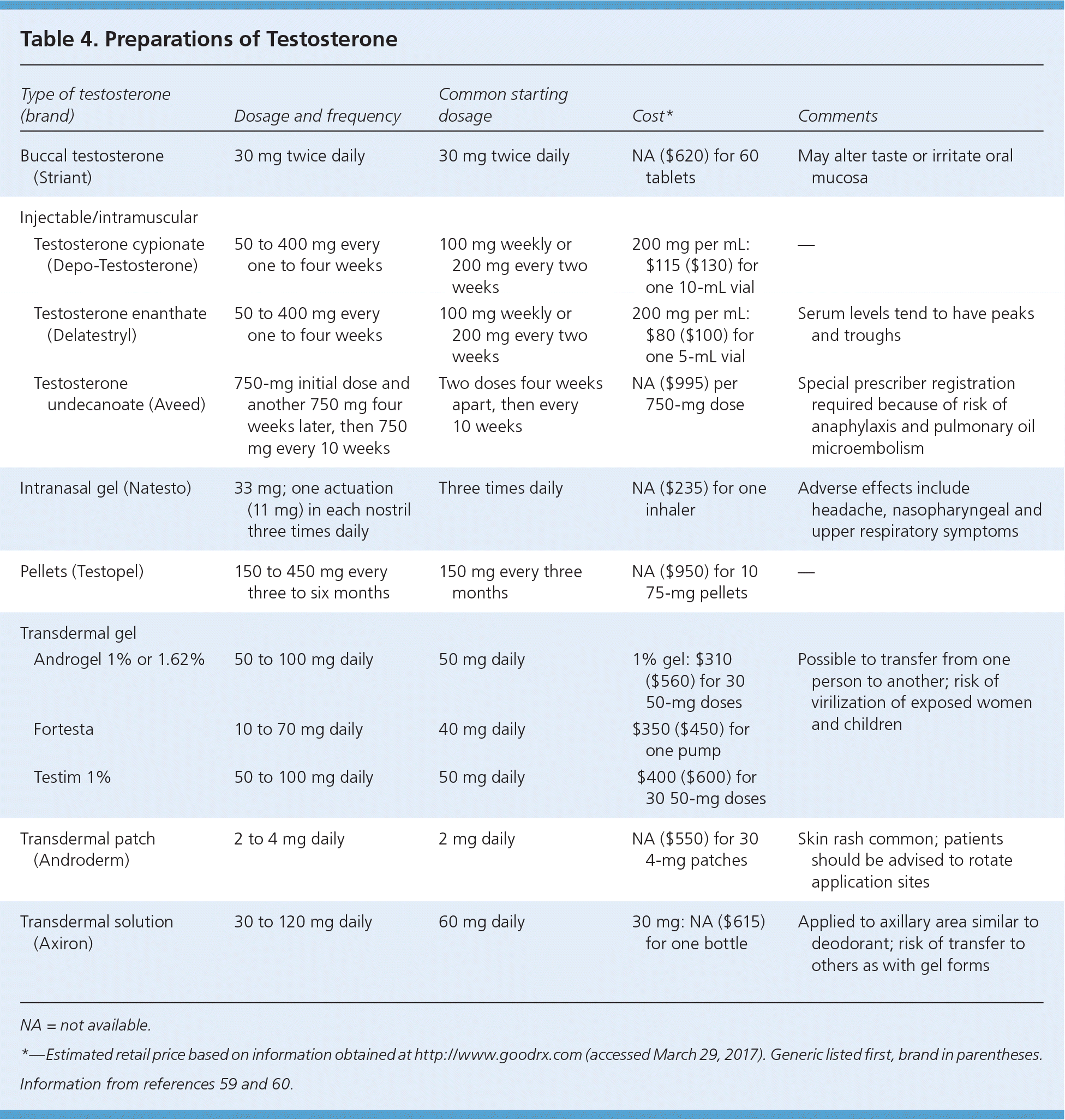
| Type of testosterone (brand) | Dosage and frequency | Common starting dosage | Cost* | Comments | |
|---|---|---|---|---|---|
| Buccal testosterone (Striant) | 30 mg twice daily | 30 mg twice daily | NA ($620) for 60 tablets | May alter taste or irritate oral mucosa | |
| Injectable/intramuscular | |||||
| Testosterone cypionate (Depo-Testosterone) | 50 to 400 mg every one to four weeks | 100 mg weekly or 200 mg every two weeks | 200 mg per mL: $115 ($130) for one 10-mL vial | — | |
| Testosterone enanthate (Delatestryl) | 50 to 400 mg every one to four weeks | 100 mg weekly or 200 mg every two weeks | 200 mg per mL: $80 ($100) for one 5-mL vial | Serum levels tend to have peaks and troughs | |
| Testosterone undecanoate (Aveed) | 750-mg initial dose and another 750 mg four weeks later, then 750 mg every 10 weeks | Two doses four weeks apart, then every 10 weeks | NA ($995) per 750-mg dose | Special prescriber registration required because of risk of anaphylaxis and pulmonary oil microembolism | |
| Intranasal gel (Natesto) | 33 mg; one actuation (11 mg) in each nostril three times daily | Three times daily | NA ($235) for one inhaler | Adverse effects include headache, nasopharyngeal and upper respiratory symptoms | |
| Pellets (Testopel) | 150 to 450 mg every three to six months | 150 mg every three months | NA ($950) for 10 75-mg pellets | — | |
| Transdermal gel | |||||
| Androgel 1% or 1.62% | 50 to 100 mg daily | 50 mg daily | 1% gel: $310 ($560) for 30 50-mg doses | Possible to transfer from one person to another; risk of virilization of exposed women and children | |
| Fortesta | 10 to 70 mg daily | 40 mg daily | $350 ($450) for one pump | ||
| Testim 1% | 50 to 100 mg daily | 50 mg daily | $400 ($600) for 30 50-mg doses | ||
| Transdermal patch (Androderm) | 2 to 4 mg daily | 2 mg daily | NA ($550) for 30 4-mg patches | Skin rash common; patients should be advised to rotate application sites | |
| Transdermal solution (Axiron) | 30 to 120 mg daily | 60 mg daily | 30 mg: NA ($615) for one bottle | Applied to axillary area similar to deodorant; risk of transfer to others as with gel forms | |
Monitoring of Men on Testosterone Therapy
Men receiving testosterone therapy should be monitored regularly for adverse effects and to ensure normalization of serum testosterone levels (Table 59 ). Before initiation of testosterone therapy, testing should include a complete blood count to measure hematocrit, and a PSA test and digital rectal examination to detect preexisting prostate cancer.9 Patients should be reevaluated for therapeutic response and adverse effects three to six months after initiation of treatment, including a repeat testosterone measurement, complete blood count, digital rectal examination, and PSA test.9,11 If laboratory results are stable, reevaluation may be performed annually.9 An increase in hematocrit to greater than 54% should lead to cessation of treatment, lowering of the dose, or change to a lower-risk formulation. An increase in PSA of greater than 1.4 ng per mL (1.4 mcg per L) over 12 months or an abnormal digital rectal examination result should prompt referral to a urologist.9
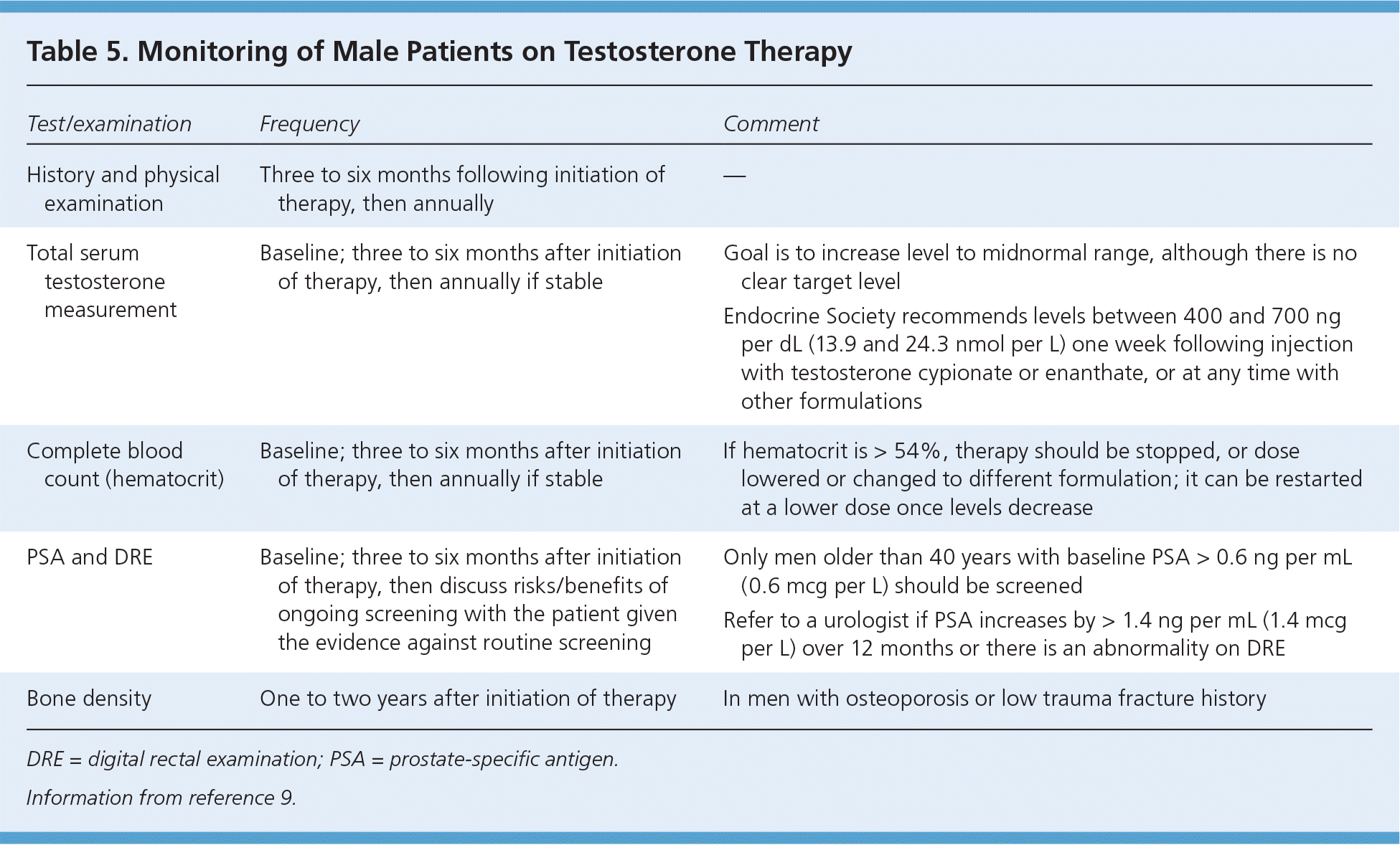
| Test/examination | Frequency | Comment |
|---|---|---|
| History and physical examination | Three to six months following initiation of therapy, then annually | — |
| Total serum testosterone measurement | Baseline; three to six months after initiation of therapy, then annually if stable | Goal is to increase level to midnormal range, although there is no clear target level |
| Endocrine Society recommends levels between 400 and 700 ng per dL (13.9 and 24.3 nmol per L) one week following injection with testosterone cypionate or enanthate, or at any time with other formulations | ||
| Complete blood count (hematocrit) | Baseline; three to six months after initiation of therapy, then annually if stable | If hematocrit is > 54%, therapy should be stopped, or dose lowered or changed to different formulation; it can be restarted at a lower dose once levels decrease |
| PSA and DRE | Baseline; three to six months after initiation of therapy, then discuss risks/benefits of ongoing screening with the patient given the evidence against routine screening | Only men older than 40 years with baseline PSA > 0.6 ng per mL (0.6 mcg per L) should be screened |
| Refer to a urologist if PSA increases by > 1.4 ng per mL (1.4 mcg per L) over 12 months or there is an abnormality on DRE | ||
| Bone density | One to two years after initiation of therapy | In men with osteoporosis or low trauma fracture history |
Of note, there is no consensus on the necessity and timing of repeated PSA testing and digital rectal examination for men on testosterone therapy. Although the Endocrine Society and a multidisciplinary Canadian panel recommend annual PSA and digital rectal examination screening in men 40 years and older,9,11 the U.S. Preventive Services Task Force recommends against routine PSA screening and does not specify its recommendation on digital rectal examination.61 Therefore, physicians and patients should engage in shared decision making, weighing the risks and benefits of ongoing prostate cancer screening in the context of testosterone therapy.
Most experts agree that the goal serum testosterone level should be in the midnormal range (i.e., 400 to 700 ng per dL [13.9 to 24.3 nmol per L]); values outside of this range require a dose adjustment.9 Most importantly, ongoing evaluation of treatment effectiveness is required.
Future and Ongoing Research
In February 2016, the first results from the Testosterone Trials sponsored by the National Institutes of Health were published.14 This set of seven randomized controlled trials assessing sexual function, vitality, physical function, cognitive function, anemia, bone density, and cardiovascular health represents the largest, most rigorously conducted study of the benefits of testosterone therapy for older men. Results of the trials assessing cognitive function, anemia, bone density, and cardiovascular health are forthcoming. However, the Testosterone Trials were designed to assess only effectiveness and not the risks of testosterone therapy, including prostate cancer or cardiovascular disease.
Testosterone Therapy in Women
In women, testosterone is produced by the ovaries and adrenal glands, and by conversion of proandrogens in peripheral tissues. Levels decrease gradually starting in the 20s or 30s. There is no abrupt decrease during menopause, with the exception of surgical menopause.62 Testosterone is also converted to estrogen by aromatases in many tissues; therefore, testosterone is an important source of estrogen in postmenopausal women.63 Testosterone deficiency in women may be associated with problems with sexual function, mood, cognition, and body composition.64
A comprehensive meta-analysis of post-menopausal women found improvement in sexual function with testosterone therapy. There was no evidence of improvement in anxiety, mood, body weight or mass, or bone density.64 Subsequently, a consensus statement released by several major organizations, including the Endocrine Society and American College of Obstetricians and Gynecologists, supported the use of testosterone therapy for hypoactive sexual desire disorder in postmenopausal women but not for any other indication.65 Of note, there are no FDA-approved products for testosterone therapy in women, and no formulations are readily available in the United States that provide the recommended treatment dosage for women (300 mcg per day), necessitating the use of compounding pharmacies. The use of testosterone therapy in women is summarized in Table 6.64,65

| Recommended only for treatment of hypoactive sexual desire disorder |
| Diagnosis is clinical; there is no established cutoff level of testosterone to indicate treatment |
| There are no formulations readily available in the United States that provide the recommended treatment dosage for women (300 mcg per day), necessitating the use of compounding pharmacies |
| Adverse effects may include virilization (acne, hirsutism, deepening of the voice) and adverse lipid changes; the effect of testosterone on breast and endometrial tissue is not well studied, although there is currently no evidence of cancer risk |
| Treatment should begin with a six-month trial period, and continued only if the patient is responding favorably at that time; there are no safety and effectiveness data beyond 24 months |
Testosterone Therapy in Transgender and Gender Diverse Patients
[corrected] Testosterone therapy may also be used to facilitate gender transition for transgender men and gender nonbinary individuals desiring masculinization. Primary care physicians are increasingly involved in the initiation and management of testosterone therapy for this population. Although a full discussion of the use of testosterone for the treatment of gender incongruence is beyond the scope of this article, physicians can find concise guidelines in the 2017 Endocrine Society publication, “Endocrine Treatment of Gender-Dysphoric/Gender-Incongruent Persons: An Endocrine Society Clinical Practice Guideline,” at https://www.endocrine.org/guidelines-and-clinical-practice/clinical-practice-guidelines/gender-dysphoria-gender-incongruence.66
This article updates a previous article on this topic by Margo and Winn.67
Data Sources: PubMed, Essential Evidence Plus, the Cochrane database, and the National Guideline Clearinghouse were searched using the key term testosterone, alone and with cardiovascular, cognition, sexual function, bone density, strength, depression, risk, benefit, and adverse event. Reference lists from the included meta-analyses were reviewed for potential sources. Search dates: November 30, 2015; January 15, 2016; February 10, 2016; and March 17, 2017.
