Am Fam Physician. 2017;96(10):668-670
Author disclosure: No relevant financial affiliations.
Key Clinical Issue
What is the accuracy of clinical decision tools and imaging for the diagnosis of gout in the primary care setting, and how effective are medications used to treat and prevent gout?
Evidence-Based Answer
The Diagnostic Rule and the Clinical Gout Diagnosis are two clinical decision tools that are 88% and 97% sensitive and 75% and 96% specific, respectively, in diagnosing gout when compared with monosodium urate crystal analysis. (Strength of Recommendation [SOR]: C, based on disease-oriented evidence.) Nonsteroidal anti-inflammatory drugs (NSAIDs), colchicine, and corticosteroids all effectively treat acute gout. (SOR: A, based on consistent, good-quality patient-oriented evidence.) Urate-lowering therapy reduces serum urate levels and frequency of gout attacks at 12 months. (SOR: B, based on inconsistent or limited-quality patient-oriented evidence.) It does not lower the frequency of gout attacks during the first six months, likely because of the increased risk of gout attacks with the initiation of therapy. Prophylactic agents such as colchicine and NSAIDs should be used during the first six months of urate-lowering therapy. (SOR: A, based on consistent, good-quality patient-oriented evidence.)
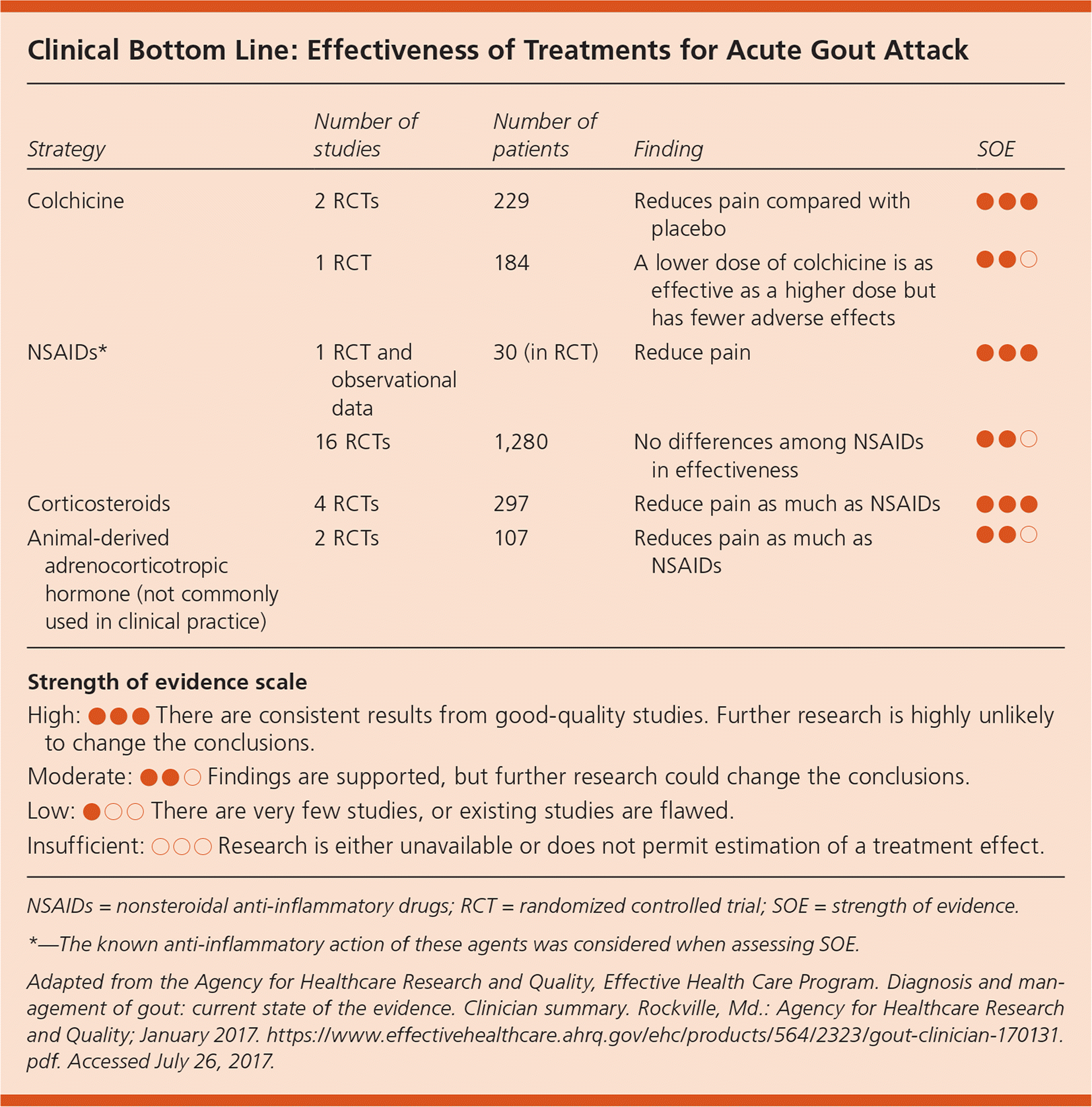
| Strategy | Number of studies | Number of patients | Finding | SOE |
|---|---|---|---|---|
| Colchicine | 2 RCTs | 229 | Reduces pain compared with placebo | ● ● ● |
| 1 RCT | 184 | A lower dose of colchicine is as effective as a higher dose but has fewer adverse effects | ● ● ○ | |
| NSAIDs* | 1 RCT and observational data | 30 (in RCT) | Reduce pain | ● ● ● |
| 16 RCTs | 1,280 | No differences among NSAIDs in effectiveness | ● ● ○ | |
| Corticosteroids | 4 RCTs | 297 | Reduce pain as much as NSAIDs | ● ● ● |
| Animal-derived adrenocorticotropic hormone (not commonly used in clinical practice) | 2 RCTs | 107 | Reduces pain as much as NSAIDs | ● ● ○ |
Strength of evidence scale | ||||
| High: ● ● ● There are consistent results from good-quality studies. Further research is highly unlikely to change the conclusions. | ||||
| Moderate: ● ● ○ Findings are supported, but further research could change the conclusions. | ||||
| Low: ● ○ ○ There are very few studies, or existing studies are flawed. | ||||
| Insufficient: ○ ○ ○ Research is either unavailable or does not permit estimation of a treatment effect. | ||||
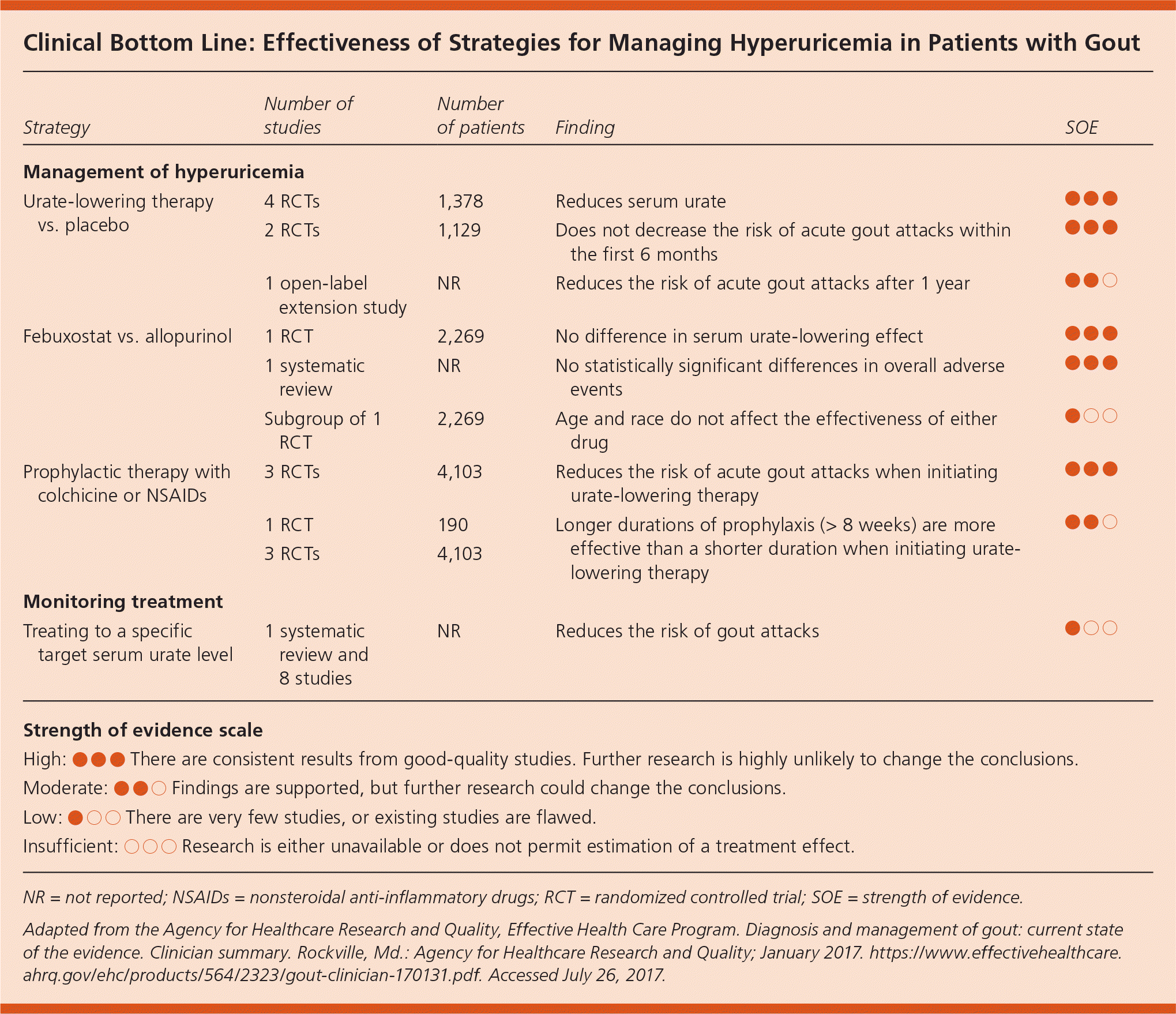
| Strategy | Number of studies | Number of patients | Finding | SOE |
|---|---|---|---|---|
| Management of hyperuricemia | ||||
| Urate-lowering therapy vs. placebo | 4 RCTs | 1,378 | Reduces serum urate | ● ● ● |
| 2 RCTs | 1,129 | Does not decrease the risk of acute gout attacks within the first 6 months | ● ● ● | |
| 1 open-label extension study | NR | Reduces the risk of acute gout attacks after 1 year | ● ● ○ | |
| Febuxostat vs. allopurinol | 1 RCT | 2,269 | No difference in serum urate-lowering effect | ● ● ● |
| 1 systematic review | NR | No statistically significant differences in overall adverse events | ● ● ● | |
| Subgroup of 1 RCT | 2,269 | Age and race do not affect the effectiveness of either drug | ● ○ ○ | |
| Prophylactic therapy with colchicine or NSAIDs | 3 RCTs | 4,103 | Reduces the risk of acute gout attacks when initiating urate-lowering therapy | ● ● ● |
| 1 RCT | 190 | Longer durations of prophylaxis (> 8 weeks) are more effective than a shorter duration when initiating urate-lowering therapy | ● ● ○ | |
| 3 RCTs | 4,103 | |||
| Monitoring treatment | ||||
| Treating to a specific target serum urate level | 1 systematic review and 8 studies | NR | Reduces the risk of gout attacks | ● ○ ○ |
Strength of evidence scale | ||||
| High: ● ● ● There are consistent results from good-quality studies. Further research is highly unlikely to change the conclusions. | ||||
| Moderate: ● ● ○ Findings are supported, but further research could change the conclusions. | ||||
| Low: ● ○ ○ There are very few studies, or existing studies are flawed. | ||||
| Insufficient: ○ ○ ○ Research is either unavailable or does not permit estimation of a treatment effect. | ||||
Practice Pointers
This Agency for Healthcare Research and Quality (AHRQ) review assessed the accuracy of clinical decision tools and imaging to diagnose gout (eTable A), as well as the treatment of gout in the primary care setting. Although the presence of monosodium urate crystals in joint aspirate remains the diagnostic standard, the Diagnostic Rule and the Clinical Gout Diagnosis decision tools predicted gout with 88% and 97% sensitivity and 75% and 96% specificity, respectively. The Clinical Gout Diagnosis tool is more accurate than the Diagnostic Rule and is based on a history of more than one attack of acute arthritis, development of maximal inflammation within one day, monoarthritis or oligoarthritis attack, redness over joints, painful or swollen first metatarsophalangeal joint, unilateral tarsal joint attack, tophi, and the presence of hyperuricemia. The sensitivity and specificity of the clinical decision tools appear to be just as good as, if not better than, imaging without the added cost or risk. Dual-energy computed tomography was 85% to 100% sensitive and 83% to 92% specific for diagnosing gout. Ultrasonography was 74% sensitive and 88% specific.1
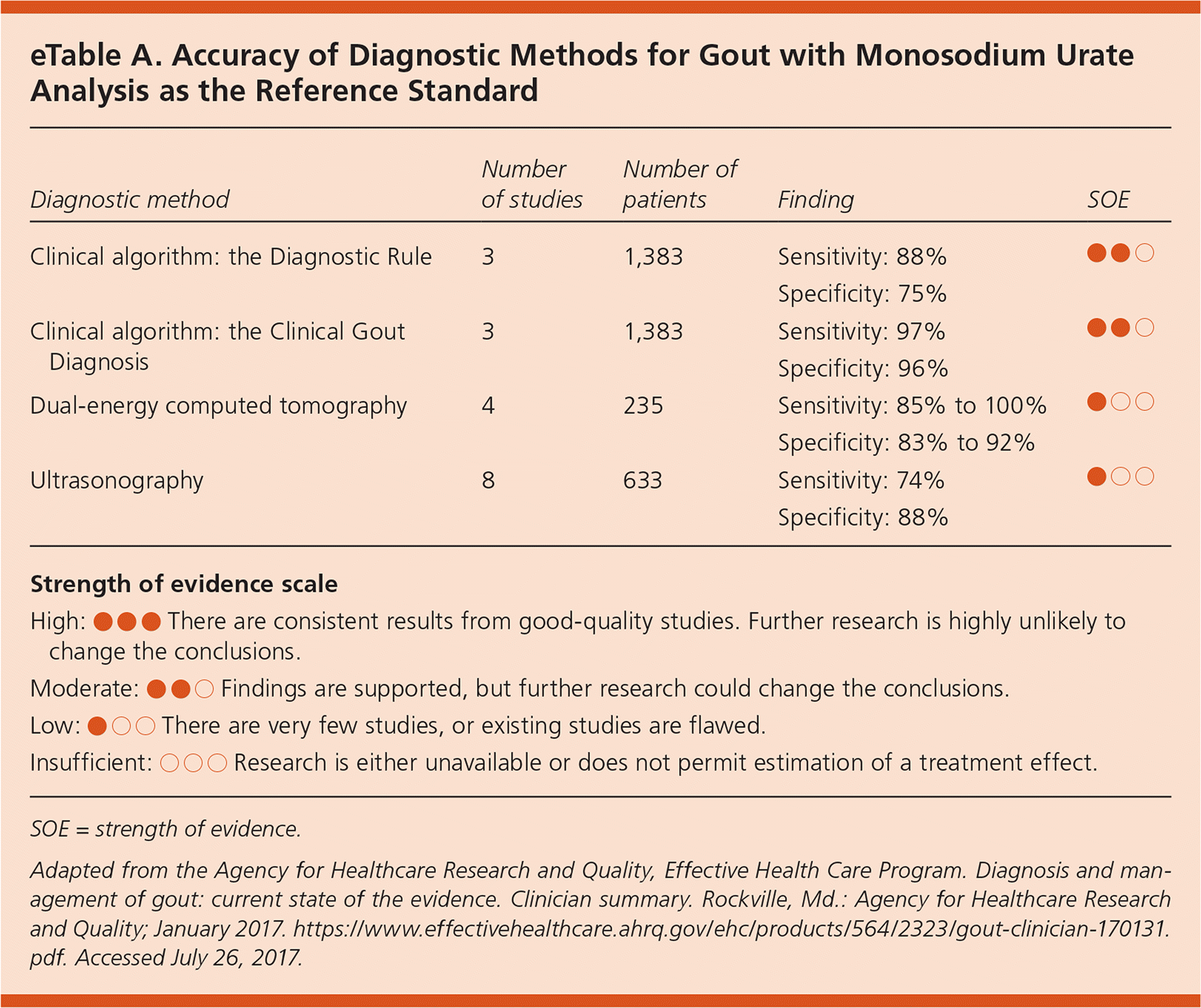
| Diagnostic method | Number of studies | Number of patients | Finding | SOE |
|---|---|---|---|---|
| Clinical algorithm: the Diagnostic Rule | 3 | 1,383 | Sensitivity: 88% | ● ● ○ |
| Specificity: 75% | ||||
| Clinical algorithm: the Clinical Gout Diagnosis | 3 | 1,383 | Sensitivity: 97% | ● ● ○ |
| Specificity: 96% | ||||
| Dual-energy computed tomography | 4 | 235 | Sensitivity: 85% to 100% | ● ○ ○ |
| Specificity: 83% to 92% | ||||
| Ultrasonography | 8 | 633 | Sensitivity: 74% | ● ○ ○ |
| Specificity: 88% | ||||
Strength of evidence scale | ||||
| High: ● ● ● There are consistent results from good-quality studies. Further research is highly unlikely to change the conclusions. | ||||
| Moderate: ● ● ○ Findings are supported, but further research could change the conclusions. | ||||
| Low: ● ○ ○ There are very few studies, or existing studies are flawed. | ||||
| Insufficient: ○ ○ ○ Research is either unavailable or does not permit estimation of a treatment effect. | ||||
NSAIDs, colchicine, and corticosteroids were all effective in reducing the pain of acute gout. Low-dose colchicine (1.2 mg initially followed by 0.6 mg one hour later) reduces pain compared with placebo, and is as effective as a higher dosage (1.2 mg followed by 0.6 mg each hour for six hours) with fewer gastrointestinal adverse effects (eTable B). Multiple NSAIDs (at variable dosages; a common comparator was indomethacin 50 mg three times daily) reduce pain with no differences among NSAIDs in terms of effectiveness. Corticosteroids (multiple agents and dosages, including a single 30-mg dose of prednisolone, prednisone 30-mg taper, and betamethasone, 7 mg intramuscularly once) also reduce pain. Allopurinol (100 to 300 mg daily) and febuxostat (20 to 240 mg daily) effectively lower serum urate levels but do not reduce the frequency of gout attacks in the first six months of therapy. The use of colchicine (0.6 mg twice daily) or NSAIDs in combination with urate-lowering therapy reduces the increased risk of gout attacks associated with initiation of urate-lowering therapy. After 12 months, urate-lowering therapy reduces the frequency of gout attacks. There is insufficient evidence to determine whether dietary and lifestyle changes are effective for managing gout; however, it is reasonable to recommend reducing dietary purines, red meat, shellfish, sugary drinks, and alcohol while encouraging weight loss and physical activity.1
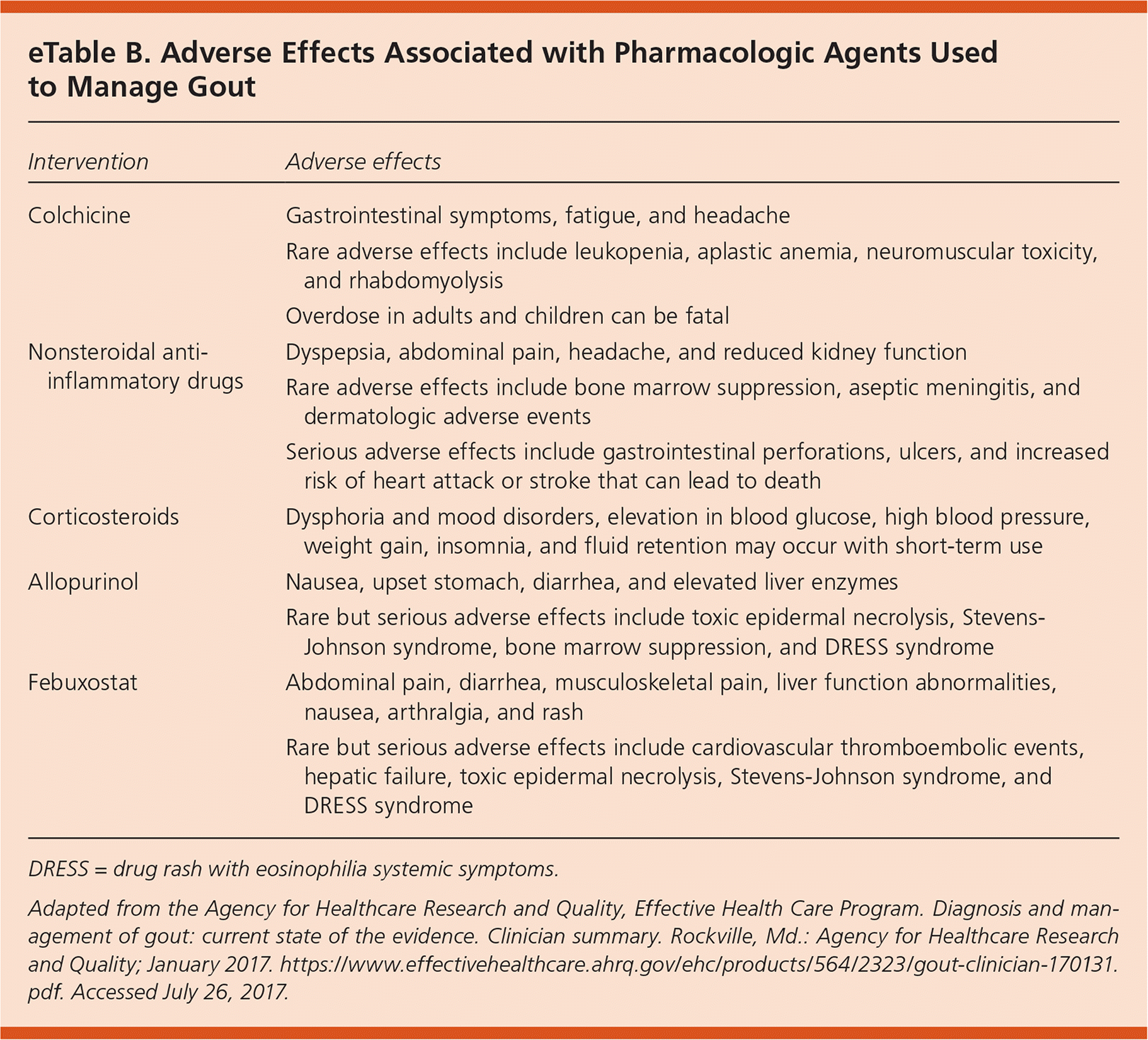
| Intervention | Adverse effects |
|---|---|
| Colchicine | Gastrointestinal symptoms, fatigue, and headache |
| Rare adverse effects include leukopenia, aplastic anemia, neuromuscular toxicity, and rhabdomyolysis | |
| Overdose in adults and children can be fatal | |
| Nonsteroidal anti-inflammatory drugs | Dyspepsia, abdominal pain, headache, and reduced kidney function |
| Rare adverse effects include bone marrow suppression, aseptic meningitis, and dermatologic adverse events | |
| Serious adverse effects include gastrointestinal perforations, ulcers, and increased risk of heart attack or stroke that can lead to death | |
| Corticosteroids | Dysphoria and mood disorders, elevation in blood glucose, high blood pressure, weight gain, insomnia, and fluid retention may occur with short-term use |
| Allopurinol | Nausea, upset stomach, diarrhea, and elevated liver enzymes |
| Rare but serious adverse effects include toxic epidermal necrolysis, Stevens-Johnson syndrome, bone marrow suppression, and DRESS syndrome | |
| Febuxostat | Abdominal pain, diarrhea, musculoskeletal pain, liver function abnormalities, nausea, arthralgia, and rash |
| Rare but serious adverse effects include cardiovascular thromboembolic events, hepatic failure, toxic epidermal necrolysis, Stevens-Johnson syndrome, and DRESS syndrome |
The American College of Physicians (ACP) used this AHRQ review to develop diagnostic and treatment guidelines with concordant conclusions: moderate evidence to support the use of clinical decision tools; low-quality evidence for dual-energy computed tomography or ultrasonography to improve diagnostic accuracy; and strong evidence for NSAIDs, corticosteroids, and colchicine for acute gout attacks.2,3 The ACP guidelines differ from those of the American College of Rheumatology, which recommend urate-lowering therapy to a serum urate level of less than 6.0 mg per dL (357 μmol per L).4,5 However, based on the AHRQ findings, the ACP guideline states that the strength of evidence is low for a specific serum urate goal.1,2 In the absence of stronger evidence supporting a specific urate goal, urate-lowering therapy may be titrated clinically to minimize gout flares.
editor's note: American Family Physician SOR ratings are different from the AHRQ Strength of Evidence ratings.
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the U.S. Army Medical Department or the U.S. Army Service at large.