Am Fam Physician. 2018;97(2):111-116
Author disclosure: No relevant financial affiliations.
Individuals at increased risk of developing colorectal cancer include those with a personal or family history of advanced adenomas or colorectal cancer, a personal history of inflammatory bowel disease, or genetic polyposis syndromes. In general, these persons should undergo more frequent or earlier testing than individuals at average risk. Individuals who have a first-degree relative with colorectal cancer or advanced adenoma diagnosed before 60 years of age or two first-degree relatives diagnosed at any age should be advised to start screening colonoscopy at 40 years of age or 10 years younger than the earliest diagnosis in their family, whichever comes first. In individuals with ulcerative colitis or Crohn disease with colonic involvement, colonoscopy should begin eight to 10 years after the onset of symptoms and be repeated every one to three years. Individuals who have a first-degree relative with hereditary nonpolyposis colorectal cancer should begin colonoscopy at 25 years of age and repeat colonoscopy every one to two years. In persons with a family history of adenomatous polyposis syndromes, screening should begin at 10 years of age or in a person's mid-20s, depending on the syndrome; repeat colonoscopy is typically required every one to two years. Screening colonoscopy should begin at eight years of age in individuals with Peutz-Jeghers syndrome. If results are normal, colonoscopy can be repeated at 18 years of age and then every three years. Persons with sessile serrated adenomatous polyposis should begin annual colonoscopy as soon as the diagnosis is established.
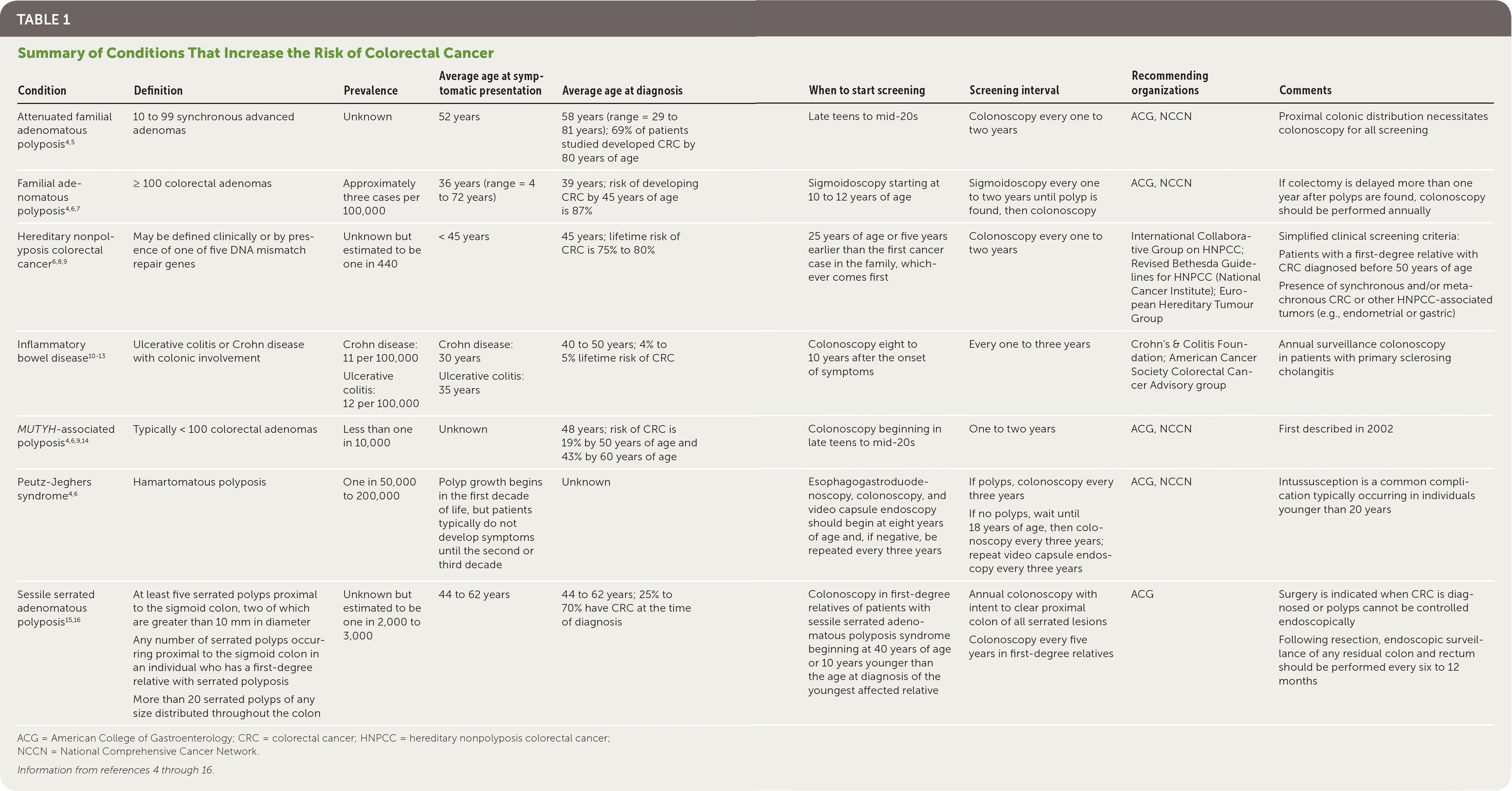
Colorectal cancer (CRC) is the third most common cancer diagnosis in the United States behind prostate and lung cancer for men and breast and lung cancer for women.1 Most organizations recommend screening average-risk individuals for CRC beginning at 50 years of age.2,3 However, it is recommended that persons at increased risk of CRC undergo more frequent or earlier testing.4 This includes individuals with a personal or family history of advanced adenomas or CRC, a personal history of inflammatory bowel disease, a risk of hereditary nonpolyposis colorectal cancer (HNPCC), or genetic polyposis syndromes (Table 14–16).
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Individuals who have a first-degree relative with colorectal cancer or advanced adenoma diagnosed before 60 years of age should start screening colonoscopy at 40 years of age or 10 years younger than the earliest diagnosis in their family, whichever comes first. If results are negative, colonoscopy should be repeated every five years. | C | 3 |
| Screening colonoscopy should begin eight to 10 years after the onset of symptoms in individuals who have Crohn disease with colonic involvement or ulcerative colitis. Screening should be repeated every one to three years. | C | 13 |
| In individuals with hereditary nonpolyposis colorectal cancer, colonoscopy should begin at 25 years of age and be repeated annually. | C | 6 |
| Individuals with adenomatous polyposis syndromes should begin colonoscopy between 10 to 20 years of age and be repeated every one to two years. | C | 4 |
| Esophagogastroduodenoscopy, colonoscopy, and video capsule endoscopy should begin at eight years of age in individuals with Peutz-Jeghers syndrome. If results are negative, testing should be repeated every three years. | C | 4 |
| In individuals with sessile serrated adenomatous polyposis, colonoscopy should begin as soon as the diagnosis is established and be repeated annually. | C | 15 |
| Condition | Definition | Prevalence | Average age at symptomatic presentation | Average age at diagnosis | When to start screening | Screening interval | Recommending organizations | Comments |
|---|---|---|---|---|---|---|---|---|
| Attenuated familial adenomatous polyposis4,5 | 10 to 99 synchronous advanced adenomas | Unknown | 52 years | 58 years (range = 29 to 81 years); 69% of patients studied developed CRC by 80 years of age | Late teens to mid-20s | Colonoscopy every one to two years | ACG, NCCN | Proximal colonic distribution necessitates colonoscopy for all screening |
| Familial adenomatous polyposis4,6,7 | ≥ 100 colorectal adenomas | Approximately three cases per 100,000 | 36 years (range = 4 to 72 years) | 39 years; risk of developing CRC by 45 years of age is 87% | Sigmoidoscopy starting at 10 to 12 years of age | Sigmoidoscopy every one to two years until polyp is found, then colonoscopy | ACG, NCCN | If colectomy is delayed more than one year after polyps are found, colonoscopy should be performed annually |
| Hereditary nonpolyposis colorectal cancer6,8,9 | May be defined clinically or by presence of one of five DNA mismatch repair genes | Unknown but estimated to be one in 440 | < 45 years | 45 years; lifetime risk of CRC is 75% to 80% | 25 years of age or five years earlier than the first cancer case in the family, whichever comes first | Colonoscopy every one to two years | International Collaborative Group on HNPCC; Revised Bethesda Guidelines for HNPCC (National Cancer Institute); European Hereditary Tumour Group | Simplified clinical screening criteria: Patients with a first-degree relative with CRC diagnosed before 50 years of age Presence of synchronous and/or metachronous CRC or other HNPCC-associated tumors (e.g., endometrial or gastric) |
| Inflammatory bowel disease10–13 | Ulcerative colitis or Crohn disease with colonic involvement | Crohn disease: 11 per 100,000 Ulcerative colitis: 12 per 100,000 | Crohn disease: 30 years Ulcerative colitis: 35 years | 40 to 50 years; 4% to 5% lifetime risk of CRC | Colonoscopy eight to 10 years after the onset of symptoms | Every one to three years | Crohn's & Colitis Foundation; American Cancer Society Colorectal Cancer Advisory group | Annual surveillance colonoscopy in patients with primary sclerosing cholangitis |
| MUTYH-associated polyposis4,6,9,14 | Typically < 100 colorectal adenomas | Less than one in 10,000 | Unknown | 48 years; risk of CRC is 19% by 50 years of age and 43% by 60 years of age | Colonoscopy beginning in late teens to mid-20s | One to two years | ACG, NCCN | First described in 2002 |
| Peutz-Jeghers syndrome4,6 | Hamartomatous polyposis | One in 50,000 to 200,000 | Polyp growth begins in the first decade of life, but patients typically do not develop symptoms until the second or third decade | Unknown | Esophagogastroduodenoscopy, colonoscopy, and video capsule endoscopy should begin at eight years of age and, if negative, be repeated every three years | If polyps, colonoscopy every three years If no polyps, wait until 18 years of age, then colonoscopy every three years; repeat video capsule endoscopy every three years | ACG, NCCN | Intussusception is a common complication typically occurring in individuals younger than 20 years |
| Sessile serrated adenomatous polyposis15,16 | At least five serrated polyps proximal to the sigmoid colon, two of which are greater than 10 mm in diameter Any number of serrated polyps occurring proximal to the sigmoid colon in an individual who has a first-degree relative with serrated polyposis More than 20 serrated polyps of any size distributed throughout the colon | Unknown but estimated to be one in 2,000 to 3,000 | 44 to 62 years | 44 to 62 years; 25% to 70% have CRC at the time of diagnosis | Colonoscopy in first-degree relatives of patients with sessile serrated adenomatous polyposis syndrome beginning at 40 years of age or 10 years younger than the age at diagnosis of the youngest affected relative | Annual colonoscopy with intent to clear proximal colon of all serrated lesions Colonoscopy every five years in first-degree relatives | ACG | Surgery is indicated when CRC is diagnosed or polyps cannot be controlled endoscopically Following resection, endoscopic surveillance of any residual colon and rectum should be performed every six to 12 months |
Currently, all organizations and guidelines recommend colonoscopy for screening or surveillance in individuals at increased risk of CRC. However, a recent meta-analysis reviewed the diagnostic accuracy of the fecal immunochemical test (FIT) for screening individuals who are at increased risk of CRC and found that FIT has good diagnostic accuracy for CRC (sensitivity = 93%; specificity = 91%; positive likelihood ratio = 10.30; negative likelihood ratio = 0.08).17 Therefore, screening with FIT may be an option in individuals at increased risk of CRC who decline colonoscopy.
Family History of Advanced Adenomas or Colorectal Cancer
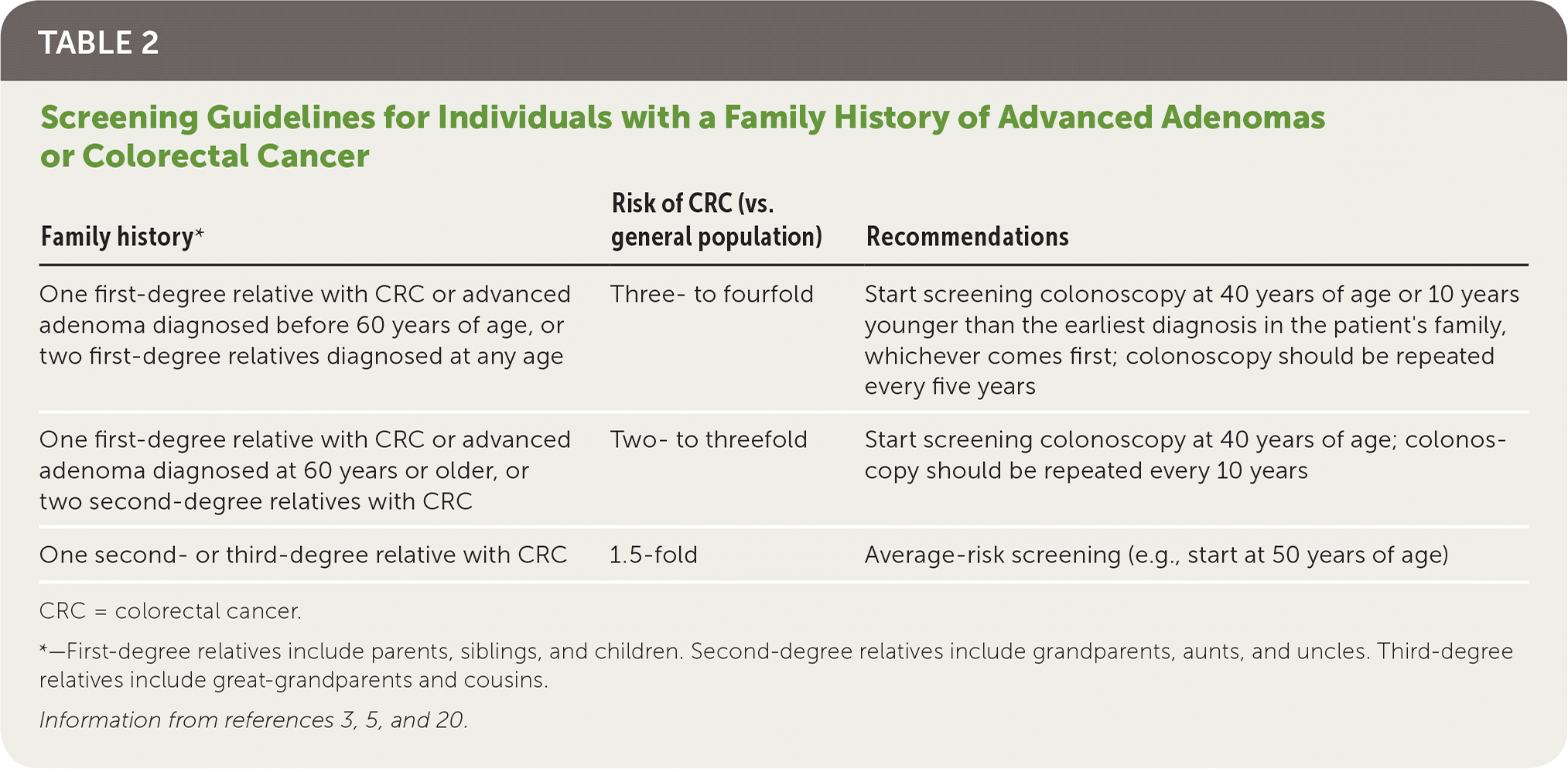
Individuals who have one first-degree relative with CRC or advanced adenoma diagnosed before 60 years of age, or two first-degree relatives diagnosed at any age, should be advised to start screening colonoscopy at 40 years of age or 10 years younger than the earliest diagnosis in their family, whichever comes first.3 Screening colonoscopy should be repeated every five years in these patients3 (Table 23,5,20).
| Family history* | Risk of CRC (vs. general population) | Recommendations |
|---|---|---|
| One first-degree relative with CRC or advanced adenoma diagnosed before 60 years of age, or two first-degree relatives diagnosed at any age | Three- to fourfold | Start screening colonoscopy at 40 years of age or 10 years younger than the earliest diagnosis in the patient's family, whichever comes first; colonoscopy should be repeated every five years |
| One first-degree relative with CRC or advanced adenoma diagnosed at 60 years or older, or two second-degree relatives with CRC | Two- to threefold | Start screening colonoscopy at 40 years of age; colonoscopy should be repeated every 10 years |
| One second- or third-degree relative with CRC | 1.5-fold | Average-risk screening (e.g., start at 50 years of age) |
Inflammatory Bowel Disease
Inflammatory bowel disease is a chronic disorder of the gastrointestinal tract that includes Crohn disease10 and ulcerative colitis.11 Individuals with inflammatory bowel disease have a 5% lifetime incidence of CRC, which is responsible for 10% to 15% of deaths in those with inflammatory bowel disease.12 Screening for CRC should begin eight to 10 years after the onset of symptoms in individuals who have Crohn disease with colonic involvement or ulcerative colitis.13 Colonoscopy should be performed during clinical remission to avoid confusing inflammatory changes with dysplasia. Regular surveillance colonoscopy should be performed after initial colonoscopy every one to three years.13 Response to therapy should not alter surveillance colonoscopy schedule.
Hereditary Nonpolyposis Colorectal Cancer
HNPCC, formerly called Lynch syndrome, is characterized by an increased risk of CRC and other malignancies, namely endometrial cancer. It accounts for 2% to 4% of all cases of CRC, and the lifetime risk of CRC in persons with HNPCC is 75% to 80%.8 HNPCC may be diagnosed clinically or by genetic testing, and the prevalence is estimated at one in 440 persons.8 CRC arises from advanced adenomas, although in HNPCC, the adenoma-carcinoma sequence is accelerated compared with sporadic CRC.9 Colonoscopy should begin at 25 years of age in persons with HNPCC, although some recommend starting at 20 years of age.6 Because of the potential for rapid progression from adenoma to carcinoma, expert consensus recommends annual colonoscopy.6
First-degree relatives of persons with HNPCC should undergo screening colonoscopy every one to two years starting at 25 years of age or five years younger than the first cancer diagnosis in the family, whichever comes first.6 Surveillance should continue until 75 years of age.6 Some families with a history of HNPCC are at increased risk of developing gastric cancer. In such families, biennial upper endoscopy should start at 50 years of age or five years younger than the age of the youngest affected relative with gastric cancer, whichever is earlier, and should continue until 75 years of age.
Adenomatous Polyposis Syndromes
FAMILIAL ADENOMATOUS POLYPOSIS
Familial adenomatous polyposis is defined as having 100 or more synchronous advanced adenomas inherited in an autosomal dominant manner.4,7 The prevalence is about three in 100,000.4 Screening with flexible sigmoidoscopy should begin around 10 years of age.4 If an adenoma is identified on sigmoidoscopy, colonoscopy should be performed.4 Colonoscopy should be performed annually if colectomy is delayed more than one year after polyps are found.4 If a genetic mutation has been found, flexible sigmoidoscopy or colonoscopy should be performed annually.6 If genetic testing has not yet been performed or is negative, annual screening should be performed until 24 years of age, then every two years until 34 years of age, then every three years until 44 years of age, and every three to five years thereafter.6 First-degree relatives of patients with familial adenomatous polyposis in whom a mutation has not been found should undergo colonoscopy,4 and upper endoscopy for gastric cancer and proximal small bowel tumors starting at 20 to 25 years of age.5
Additional screening may include annual thyroid examination on physical examination and possibly ultrasonography to screen for malignancy and upper endoscopy for gastric cancer and proximal small bowel tumors starting at 20 to 25 years of age.5 This applies to all adenomatous polyposis syndromes.
ATTENUATED FAMILIAL ADENOMATOUS POLYPOSIS
Attenuated familial adenomatous polyposis is defined as 10 to 99 synchronous advanced adenomas with a proximal colonic distribution inherited in an autosomal dominant manner.4 The exact prevalence is unknown.4 Screening colonoscopy should begin in the late teens to mid-20s and be performed every one to two years.4
MUTYH-ASSOCIATED POLYPOSIS
MUTYH-associated polyposis is caused by a mutation in the MUTYH gene and is defined as typically fewer than 100 advanced adenomas inherited in an autosomal recessive pattern.6 The prevalence is less than one in 10,000, although the incidence is unknown.9 CRC presents in patients with MUTYH-associated polyposis between 50 to 70 years of age with a 100% lifetime risk of CRC.9 Colonoscopy should begin in the late teens to mid 20s, with colonoscopy every one to two years.6 The timing of surgery should be individualized, but if polyps become endoscopically uncontrollable, then colectomy is indicated.4 Upper endoscopy to screen for gastric and duodenal polyps should be offered starting between 20 and 25 years of age and then every one to five years.9,14
Peutz-Jeghers Syndrome
Peutz-Jeghers syndrome presents as hamartomatous polyps of the gastrointestinal tract and mucocutaneous pigmentation (usually on the lips, buccal mucosa, and periorbital area) and is inherited in an autosomal dominant pattern.4,6 The prevalence of Peutz-Jeghers syndrome is estimated at one in 50,000 to 200,000.4 Esophagogastroduodenoscopy (EGD), colonoscopy, and video capsule endoscopy should begin at eight years of age.4 If any polyps are found, EGD and colonoscopy should be repeated every three years.4 If no polyps are identified, EGD and colonoscopy should be repeated at 18 years of age and every three years thereafter.4 The goal of treatment is the removal of all polyps; however, this is not always achievable, and colectomy may be necessary in some patients.4 Extracolonic cancer screening includes magnetic resonance cholangiopancreatography or endoscopic ultrasonography of the pancreas every one to two years beginning at 30 to 35 years of age; annual mammography and breast magnetic resonance imaging beginning at 25 years of age in women; annual pelvic examination and Papanicolaou smear beginning at 18 to 20 years of age in women; and annual testicular examination beginning at 10 years of age in males.4,6
Sessile Serrated Adenomatous Polyposis
Although a definitive genetic mutation has not been identified, sessile serrated adenomatous polyposis demonstrates the hallmarks of a genetic disease. It is defined as (1) at least five serrated polyps proximal to the sigmoid colon, two of which are greater than 10 mm in diameter; (2) any number of serrated polyps occurring proximal to the sigmoid colon in an individual who has a first-degree relative with the condition; and (3) more than 20 serrated polyps of any size distributed throughout the colon.15 The actual prevalence is unknown but is estimated to be one in 2,000 to 3,000.15 Patients meeting the World Health Organization criteria who elect long-term endoscopic surveillance should receive annual colonoscopy after the diagnosis is established, although this interval may be individualized based on the extent of the polyp burden.15 Surgery is indicated when CRC is diagnosed or the number of polyps makes endoscopic control unfeasible.15 Following resection, endoscopic surveillance of any residual colon and rectum should be performed every six to 12 months.16 It is recommended that screening colonoscopy be performed in first-degree relatives of patients with sessile serrated adenomatous polyposis beginning at 40 years of age or 10 years younger than the age at diagnosis of the youngest affected relative. Colonoscopy should be repeated at five-year intervals or more frequently if polyps are found.15
Data Sources: A clinical librarian completed a general PubMed search using the following MeSH terms: irritable bowel syndrome, colonoscopy, colorectal neoplasms, adenomatous polyp, familial adenomatous polyposis, MUTYH-associated polyposis, colorectal adenomatous polyposis, autosomal recessive, Peutz-Jeghers syndrome, and hereditary nonpolyposis. These terms were also used as keywords in a number of combinations. The search included meta-analyses, randomized controlled trials, and practice guidelines within the previous 20 years and was expanded to reviews and clinical trials where needed. Reviews were hand-searched for further articles. Also searched were the Cochrane databases and Essential Evidence Plus. Search dates: March 2016 and May 2017.