The online version of this article has been updated to incorporate recommendations from the SHEA/IDSA clinical practice guideline published on February 15, 2018.
Am Fam Physician. 2018;97(3):196-199
Author disclosure: No relevant financial affiliations.
Key Clinical Issue
How effective are interventions to prevent and treat Clostridium difficile infection?
Evidence-Based Answer
Antibiotic stewardship and handwashing campaigns reduce C. difficile infection without reported harms. (Strength of Recommendation [SOR]: B, based on inconsistent or limited-quality patient-oriented evidence.) Vancomycin has a higher initial cure rate than metronidazole, although the recurrence rate is equal between the two drugs. Fidaxomicin has a lower recurrence rate than vancomycin, although there is no difference in the initial cure rate. (SOR: B, based on inconsistent or limited-quality patient-oriented evidence.) There is low strength but consistent evidence that Lactobacillus, multiorganism probiotics, and fecal microbiota transplantation are effective in reducing C. difficile infection recurrence.1 (SOR: B, based on inconsistent or limited-quality patient-oriented evidence.)
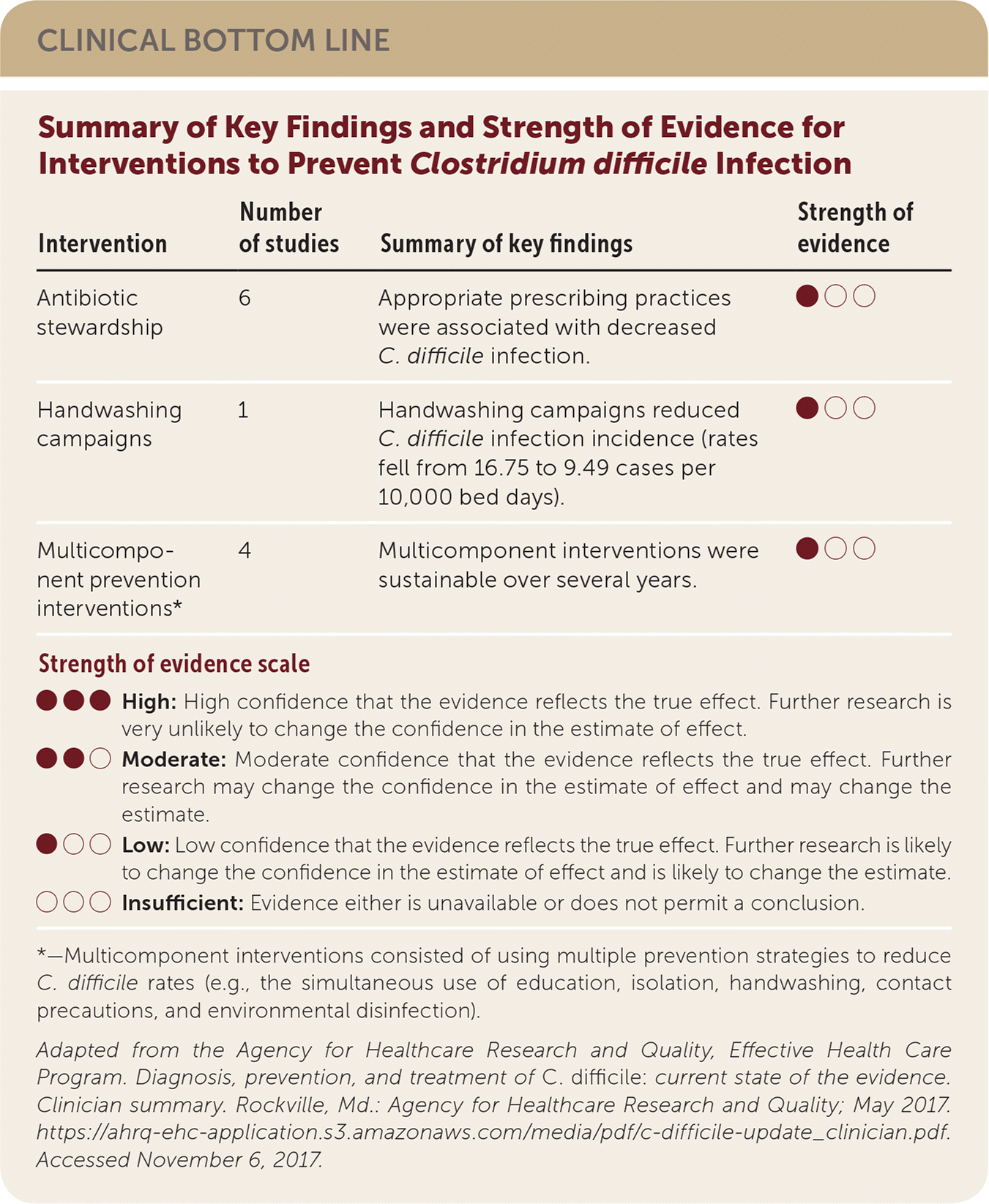
| Intervention | Number of studies | Summary of key findings | Strength of evidence |
|---|---|---|---|
| Antibiotic stewardship | 6 | Appropriate prescribing practices were associated with decreased C. difficile infection. | ●○○ |
| Handwashing campaigns | 1 | Handwashing campaigns reduced C. difficile infection incidence (rates fell from 16.75 to 9.49 cases per 10,000 bed days). | ●○○ |
| Multicomponent prevention interventions* | 4 | Multicomponent interventions were sustainable over several years. | ●○○ |
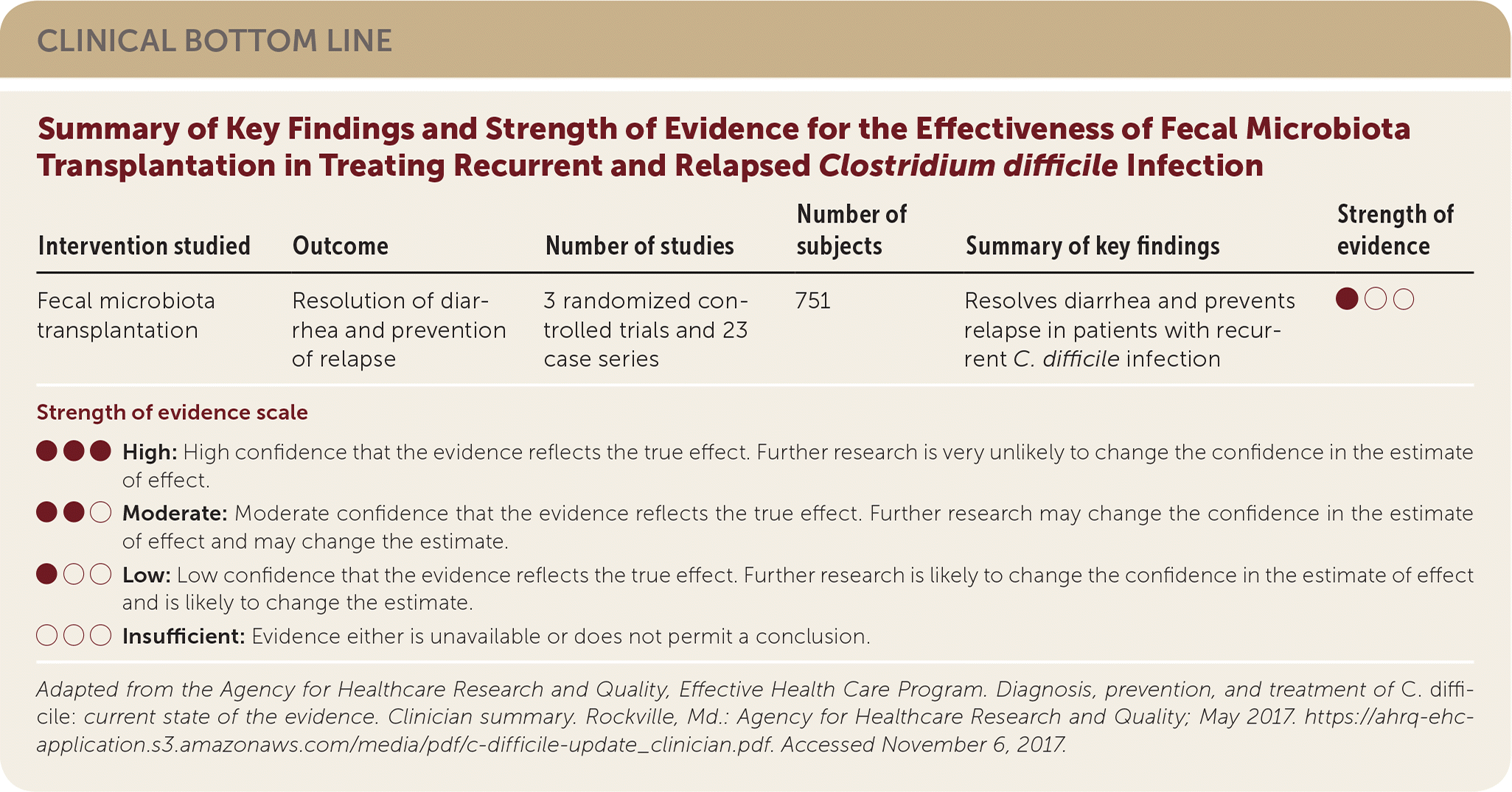
| Intervention studied | Outcome | Number of studies | Number of subjects | Summary of key findings | Strength of evidence |
|---|---|---|---|---|---|
| Fecal microbiota transplantation | Resolution of diarrhea and prevention of relapse | 3 randomized controlled trials and 23 case series | 751 | Resolves diarrhea and prevents relapse in patients with recurrent C. difficile infection | ●○○ |
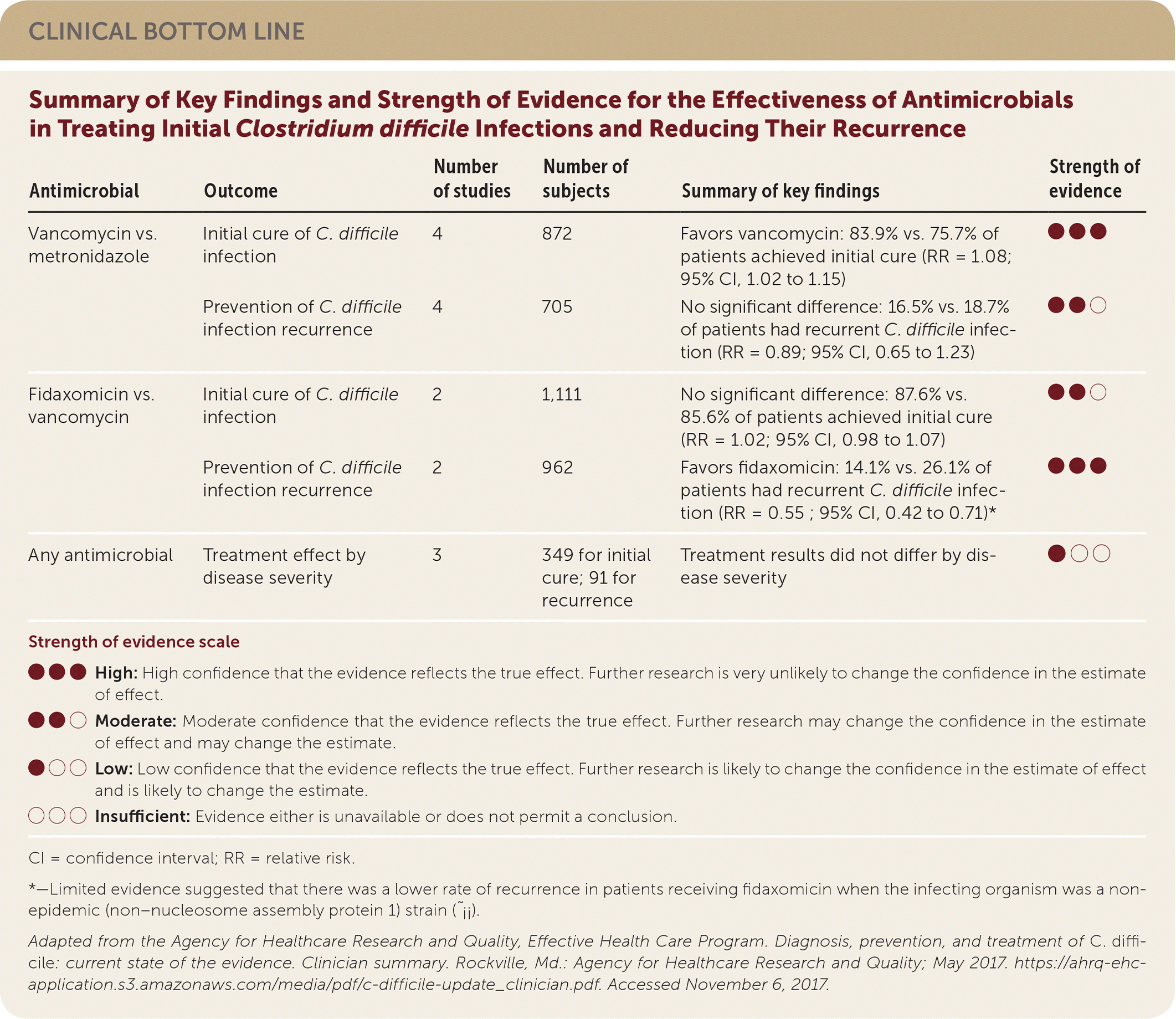
| Antimicrobial | Outcome | Number of studies | Number of subjects | Summary of key findings | Strength of evidence |
|---|---|---|---|---|---|
| Vancomycin vs. metronidazole | Initial cure of C. difficile infection | 4 | 872 | Favors vancomycin: 83.9% vs. 75.7% of patients achieved initial cure (RR = 1.08; 95% CI, 1.02 to 1.15) | ●●● |
| Prevention of C. difficile infection recurrence | 4 | 705 | No significant difference: 16.5% vs. 18.7% of patients had recurrent C. difficile infection (RR = 0.89; 95% CI, 0.65 to 1.23) | ●●○ | |
| Fidaxomicin vs. vancomycin | Initial cure of C. difficile infection | 2 | 1,111 | No significant difference: 87.6% vs. 85.6% of patients achieved initial cure (RR = 1.02; 95% CI, 0.98 to 1.07) | ●●○ |
| Prevention of C. difficile infection recurrence | 2 | 962 | Favors fidaxomicin: 14.1% vs. 26.1% of patients had recurrent C. difficile infection (RR = 0.55_; 95% CI, 0.42 to 0.71)* | ●●● | |
| Any antimicrobial | Treatment effect by disease severity | 3 | 349 for initial cure; 91 for recurrence | Treatment results did not differ by disease severity | ●○○ |
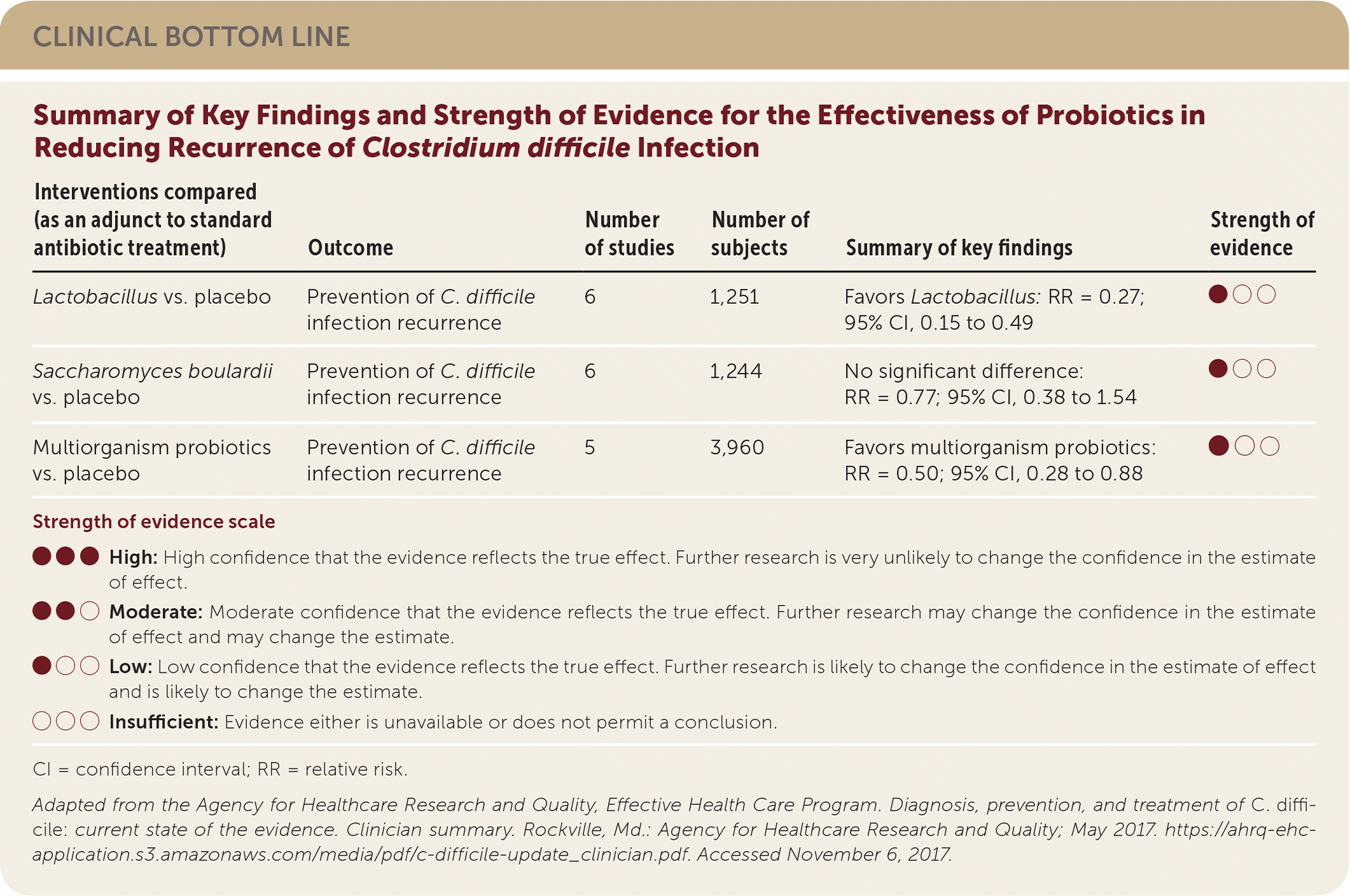
| Interventions compared (as an adjunct to standard antibiotic treatment) | Outcome | Number of studies | Number of subjects | Summary of key findings | Strength of evidence |
|---|---|---|---|---|---|
| Lactobacillus vs. placebo | Prevention of C. difficile infection recurrence | 6 | 1,251 | Favors Lactobacillus: RR = 0.27; 95% CI, 0.15 to 0.49 | ●○○ |
| Saccharomyces boulardii vs. placebo | Prevention of C. difficile infection recurrence | 6 | 1,244 | No significant difference: RR = 0.77; 95% CI, 0.38 to 1.54 | ●○○ |
| Multiorganism probiotics vs. placebo | Prevention of C. difficile infection recurrence | 5 | 3,960 | Favors multiorganism probiotics: RR = 0.50; 95% CI, 0.28 to 0.88 | ●○○ |
Practice Pointers
There are an estimated 350,000 C. difficile infection–related hospitalizations in the United States every year with approximately 9% ending in death. The number of patients discharged from the hospital with any C. difficile–related diagnosis has more than doubled since the 1990s.2 In 2008, C. difficile infection was estimated to account for $4.8 billion in health care expenses.3
This Agency for Healthcare Research and Quality (AHRQ) review update included 93 articles published between 2010 and April 2015. Based on a systematic review that included one randomized controlled trial (RCT) and five interrupted time series studies, antibiotic stewardship is associated with decreased C. difficile infection. No studies found harms associated with antibiotic stewardship. Handwashing campaigns reduced C. difficile infection rates from 16.8 to 9.5 cases per 10,000 bed days. There is insufficient evidence to determine the effectiveness of chlorhexidine gluconate baths, hydrogen peroxide vapor with terminal room cleaning, daily cleanings with hydrogen peroxide disposable wipes, and pulsed xenon ultraviolet light after terminal room cleaning. Fifteen single-hospital studies assessed multicomponent prevention strategies. Although there is insufficient evidence to assess the effectiveness of these strategies, four studies demonstrated that they are sustainable over time.1
Regarding treatment, this AHRQ review found high strength of evidence based on four RCTs that oral vancomycin has higher initial C. difficile infection cure rates compared with metronidazole (83.9% vs. 75.7%; number needed to treat [NNT] = 12; 95% confidence interval [CI], 7 to 35), and no difference between fidaxomicin and vancomycin in initial cure. When assessing C. difficile infection recurrence rates, fidaxomicin is favored over vancomycin (14.1% vs. 26.1%; NNT = 8; 95% CI, 6 to 15), and there is no difference between vancomycin and metronidazole. Notably, two RCT subgroup analyses found that differences in disease severity did not significantly affect the results.1
Three RCTs and 23 case series described the use of fecal microbiota transplantation. In most studies, it was performed after antimicrobial therapy. Fecal microbiota transplantation decreased recurrence rates, although studies were small (N < 100), and follow-up time varied from three weeks to eight years.1 A recent editorial in American Family Physician reviewed the evidence and indications for fecal microbiota transplantation (http://www.aafp.org/afp/2017/0315/p351.html).
Seventeen RCTs assessed probiotics as an adjunct to standard antibiotic treatment for the prevention of C. difficile infection. Both Lactobacillus (NNT = 26; 95% CI, 16 to 58) and multiorganism probiotics (NNT = 91; 95% CI, 52 to 294) are superior to placebo at preventing C. difficile infection, but Saccharomyces species are not.1
Guidelines from the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA) are mostly consistent with the findings in this review. SHEA/IDSA recommend that clinicians prescribe vancomycin or fidaxomicin for initial treatment of C. difficile, and use fecal microbiota transplantation for patients with multiple recurrences or inadequate response to antibiotics. However, they conclude that data is insufficient to recommend probiotics for primary prevention of C. difficile infection4 [updated]
The U.S. Food and Drug Administration recently approved bezlotoxumab, the first human monoclonal antibody treatment for C. difficile infection. A single intravenous dose of bezlotoxumab added to the standard treatment course of metronidazole, vancomycin, or fidaxomicin was found to decrease C. difficile infection recurrence rates in two industry-sponsored RCTs.5
C. difficile infection is a common problem that causes substantial health and financial burdens. Based on this AHRQ review, physicians should focus on handwashing and antibiotic stewardship as first-line interventions to prevent the illness. Clinicians may also consider recommending Lactobacillus or multiorganism probiotics for patients using antibiotics, especially in those older than 65 years because they are at the highest risk of complication from C. difficile infection.2 Despite the slightly higher cure rate of oral vancomycin, clinicians may want to start treatment with metronidazole because it is more cost-effective and there is no difference in recurrence rates.
The associated AHRQ product was funded under Contract No. HHSA 290-2012-00016-I Task Order 12 from the Agency for Healthcare Research and Quality (AHRQ), U.S. Department of Health and Human Services (HHS). The authors of this manuscript are responsible for its content. Statements in the manuscript do not necessarily represent the official views of or imply endorsement by AHRQ or HHS.
Editor's Note: American Family Physician SOR ratings are different from the AHRQ Strength of Evidence (SOE) ratings.