
Am Fam Physician. 2018;97(4):261-268
Patient information: See related handout on testicular cancer.
Author disclosure: No relevant financial affiliations.
Testicular cancer is the most common solid tumor among males 15 to 34 years of age, with an estimated 8,850 new cases and 410 deaths during 2017 in the United States. With effective treatment, the overall five-year survival rate is 97%. Risk factors for testicular cancer include undescended testis (cryptorchidism), personal or family history of testicular cancer, age, ethnicity, and infertility. The U.S. Preventive Services Task Force recommends against routine screening in asymptomatic men. Men with symptoms should receive a complete history and physical examination. Scrotal ultrasonography is the preferred initial imaging study. If a solid intratesticular mass is discovered, orchiectomy is both diagnostic and therapeutic. Staging through chest radiography, chemistry panel, liver function tests, and tumor markers guides treatment. Active surveillance, chemotherapy, retroperitoneal lymph node dissection, and radiation therapy are treatment options following orchiectomy. For patients desiring future fertility, sperm banking should be discussed early in the course of treatment. Family physicians often play a role in the care of cancer survivors and should be familiar with monitoring for recurrence and future complications, including secondary malignant neoplasms, cardiovascular risk, and infertility and subfertility.
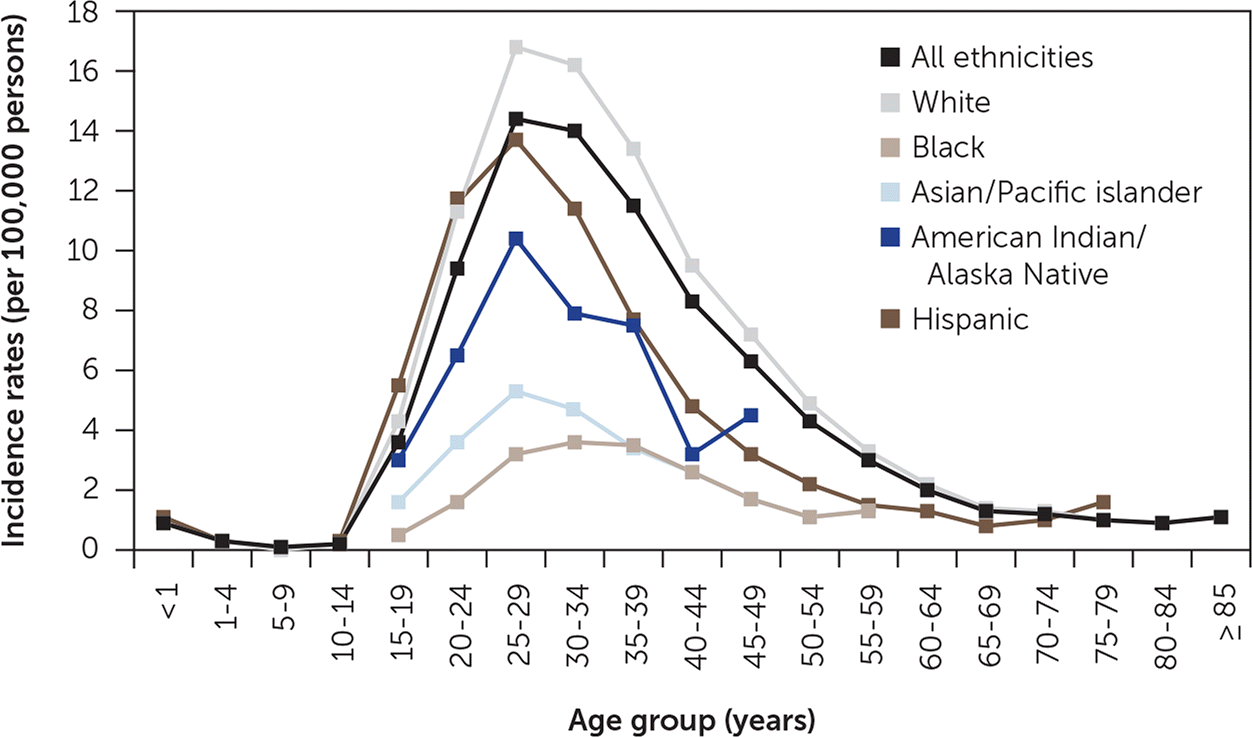
Testicular cancer is the most common solid tumor among males 15 to 34 years of age. The age-adjusted annual incidence in the United States is 5.6 cases per 100,000 persons, with a peak of 14.6 cases per 100,000 persons 30 to 34 years of age. Figure 1 includes incidence rates by age and ethnicity.1 In 2017, there were an estimated 8,850 new cases of testicular cancer and 410 deaths. Whites, Hispanics, and American Indian/Alaska Natives have the highest rates of testicular cancer.1 The incidence of testicular cancer has increased over the past several decades for unclear reasons. With effective treatment, the overall five-year survival rate is 97%.2
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Routine screening for testicular cancer in asymptomatic men is not recommended. | C | 18, 19, 21 |
| Confirmation of an alternative diagnosis is required to exclude testicular cancer in patients with a scrotal mass. | C | 24–26 |
| Scrotal ultrasonography is the preferred initial imaging study for evaluating a testicular mass. | C | 24–26 |
| Active surveillance (orchiectomy without additional therapy) is a reasonable treatment option for stage I germ cell tumors with no risk factors for relapse. | B | 24–26, 33, 34, 37, 39 |
| After primary treatment for testicular cancer, primary care physicians should routinely monitor patients for recurrence, secondary malignancy, infertility, cardiovascular disease, and other complications of chemotherapy and radiotherapy. | C | 24–26, 43, 47–51, 53–55 |
| For patients desiring future fertility, sperm banking should be discussed early in the course of treatment. | C | 24–26, 47 |

| Germ cell tumors (95% of all testicular cancers) | ||
| Derived from germ cell neoplasia in situ | ||
Seminoma | ||
Nonseminoma (nonseminomatous germ cell tumors) | ||
Embryonal carcinoma | ||
Yolk sac tumor (postpubertal) | ||
Trophoblastic tumors (e.g., choriocarcinoma, placental site trophoblastic tumor) | ||
Teratoma (postpubertal) with or without malignant transformation | ||
| Mixed and unclassified germ cell tumors | ||
| Not derived from germ cell neoplasia in situ | ||
Spermatocytic tumor | ||
Teratoma (prepubertal) | ||
Yolk sac tumor (prepubertal) | ||
| Sex cord–stromal tumors (< 5% of all testicular cancers) | ||
| Leydig cell tumor | ||
| Sertoli cell tumor | ||
| Granulosa cell tumor | ||
| Mixed and unclassified sex cord–stromal tumors | ||
| Mixed germ cell and stromal tumors (proportion of all testicular cancers not well defined) | ||
| Gonadoblastoma | ||
| Miscellaneous tumors (proportion of all testicular cancers not well defined) | ||
| Ovarian epithelial-type tumors | ||
| Hemangioma | ||
| Hematolymphoid tumors | ||
| Tumors of the collecting duct and rete testis (adenocarcinoma) | ||
Risk Factors
In patients with cryptorchidism, the relative risk of developing testicular cancer ranges from 2.9 to 6.3; the risk is increased in both testes, although the risk is much higher in the ipsilateral testis (6.3 vs. 1.7).4,5 Among these patients, the risk of cancer increases when orchiopexy is delayed until after puberty or never performed (odds ratio = 5.8) compared with early orchiopexy.6,7 Even after early orchiopexy, the risk of testicular cancer remains elevated (relative risk = 2.2) compared with the general population.8
Patients with a personal history of testicular cancer have a 12-times greater risk of developing a contralateral testicular cancer than the general population. However, the greatest risk is in the first five years after diagnosis, and the 15-year cumulative risk is 1.9%.9 Patients with a father or brother with testicular cancer have a 3.8- and 8.6-times greater risk, respectively.10
Men with infertility have an increased risk of testicular cancer, with a standardized incidence ratio of 1.6 to 2.8, although the underlying mechanism is unclear.11–13 Human immunodeficiency virus infection/AIDS increases the risk of seminoma, but this is negated with highly active antiretroviral treatment.14 Associations between testicular cancer and marijuana use, inguinal hernia, diet, maternal smoking, and body size are inconclusive.15–17 Testicular microlithiasis, vasectomy, and scrotal trauma are not risk factors for testicular cancer.16
Screening Recommendations
The U.S. Preventive Services Task Force, National Cancer Institute, and American Academy of Family Physicians recommend against screening for testicular cancer (by a clinician or through self-examination) in asymptomatic adolescents and adults because of its low incidence and high survival rate.18–20 The American Cancer Society states that a testicular examination should be part of a routine cancer-related checkup but does not include a recommendation on regular testicular self-examinations for all men.21
Diagnosis
CLINICAL PRESENTATION

| Scrotal | Metastases |
|---|---|
| Acute pain in the testis or scrotum | Gastrointestinal symptoms |
| Dull ache in the scrotum or abdomen | Gynecomastia |
| Firmness of the testis | Headaches |
| Painless, solid testicular mass | Low back pain |
| Scrotal heaviness | Neck mass |
| Scrotal swelling | Respiratory symptoms (cough, dyspnea, hemoptysis) |
Testicular changes may be detected by the patient or by a sex partner. Epididymitis is an important part of the differential diagnosis of a scrotal mass. The management of epididymitis was previously reviewed in American Family Physician (http://www.aafp.org/afp/2016/1101/p723.html). If tenderness, swelling, or examination abnormalities persist after antibiotic treatment, further evaluation is necessary.23 Confirmation of an alternative diagnosis is required to exclude testicular cancer in patients with a scrotal mass.24–26
HISTORY AND PHYSICAL EXAMINATION
Evaluation should be guided by a complete history of the presenting symptoms and assessment for risk factors. Testicular examination should include the affected and unaffected testis for comparison. The normal testis is 3.5 to 5 cm in length, smooth, homogenous, movable, and detached from the epididymis. Hard, firm, or fixed areas within or adjacent to the testes are abnormal and warrant further evaluation. Physical examination should also include evaluation of the inguinal and supra-clavicular lymph nodes, the abdomen, and the chest for gynecomastia (related to tumor secretion of beta human chorionic gonadotropin).27
IMAGING
Scrotal ultrasonography is the preferred initial imaging study for evaluating a testicular mass.24–26 It can confirm the presence of a mass, determine intra- vs. extratesticular location, and explore the contralateral testis. Ultrasonography has a sensitivity of 92% to 98% and specificity of 95% to 99.8%.28 The cost of magnetic resonance imaging offsets any advantage in specificity in most cases.29 A solid intratesticular mass on ultrasonography warrants rapid referral for radical inguinal orchiectomy because this procedure provides pathologic diagnosis and is the cornerstone of treatment.
STAGING
Before orchiectomy, evaluation includes a chemistry panel, liver function tests, and tumor markers, including measurement of beta human chorionic gonadotropin, lactate dehydrogenase (may be elevated in seminoma), and alpha fetoprotein (may be elevated in nonseminoma). Tumor markers are rechecked after orchiectomy because staging is based on postorchiectomy values. Chest radiography and abdominal/pelvic computed tomography (CT) should be performed to assess for metastasis. If abnormalities are noted, chest CT should be added. Brain magnetic resonance imaging or a bone scan may be considered if there is concern for metastasis on history and physical examination.25,26
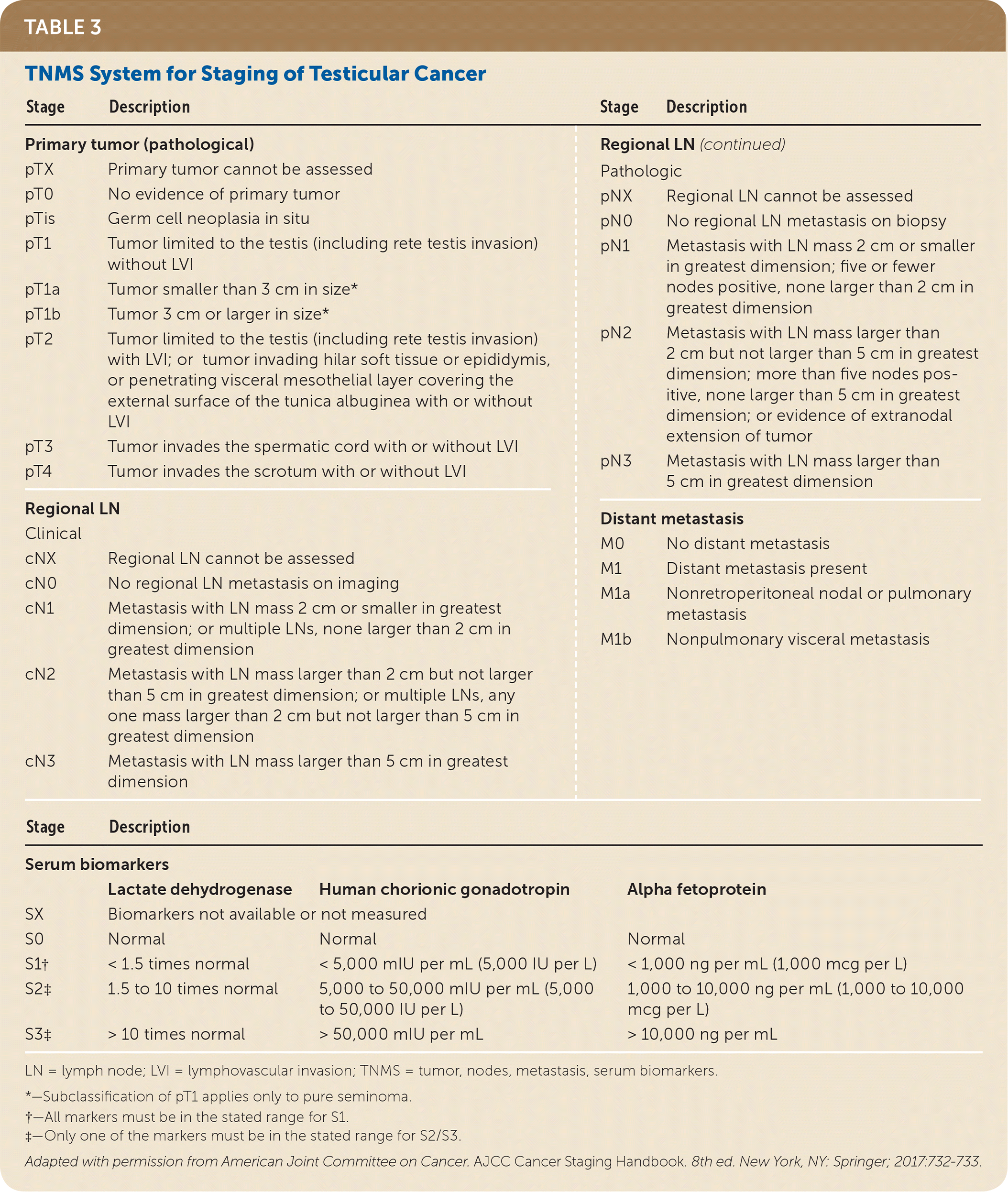
| Stage | Description | Stage | Description | |
| Primary tumor (pathological) | Regional LN (continued) | |||
| pTX | Primary tumor cannot be assessed | Pathologic | ||
| pT0 | No evidence of primary tumor | pNX | Regional LN cannot be assessed | |
| pTis | Germ cell neoplasia in situ | pN0 | No regional LN metastasis on biopsy | |
| pT1 | Tumor limited to the testis (including rete testis invasion) without LVI | pN1 | Metastasis with LN mass 2 cm or smaller in greatest dimension; five or fewer nodes positive, none larger than 2 cm in greatest dimension | |
| pT1a | Tumor smaller than 3 cm in size* | pN2 | Metastasis with LN mass larger than 2 cm but not larger than 5 cm in greatest dimension; more than five nodes positive, none larger than 5 cm in greatest dimension; or evidence of extranodal extension of tumor | |
| pT1b | Tumor 3 cm or larger in size* | pN3 | Metastasis with LN mass larger than 5 cm in greatest dimension | |
| pT2 | Tumor limited to the testis (including rete testis invasion) with LVI; or tumor invading hilar soft tissue or epididymis, or penetrating visceral mesothelial layer covering the external surface of the tunica albuginea with or without LVI | Distant metastasis | ||
| pT3 | Tumor invades the spermatic cord with or without LVI | M0 | No distant metastasis | |
| pT4 | Tumor invades the scrotum with or without LVI | M1 | Distant metastasis present | |
| Regional LN | M1a | Nonretroperitoneal nodal or pulmonary metastasis | ||
| Clinical | M1b | Nonpulmonary visceral metastasis | ||
| cNX | Regional LN cannot be assessed | |||
| cN0 | No regional LN metastasis on imaging | |||
| cN1 | Metastasis with LN mass 2 cm or smaller in greatest dimension; or multiple LNs, none larger than 2 cm in greatest dimension | |||
| cN2 | Metastasis with LN mass larger than 2 cm but not larger than 5 cm in greatest dimension; or multiple LNs, any one mass larger than 2 cm but not larger than 5 cm in greatest dimension | |||
| cN3 | Metastasis with LN mass larger than 5 cm in greatest dimension | |||
| Stage | Description | |||
| Serum biomarkers | ||||
| Lactate dehydrogenase | Human chorionic gonadotropin | Alpha fetoprotein | ||
| SX | Biomarkers not available or not measured | |||
| S0 | Normal | Normal | Normal | |
| S1† | < 1.5 times normal | < 5,000 mIU per mL (5,000 IU per L) | < 1,000 ng per mL (1,000 mcg per L) | |
| S2‡ | 1.5 to 10 times normal | 5,000 to 50,000 mIU per mL (5,000 to 50,000 IU per L) | 1,000 to 10,000 ng per mL (1,000 to 10,000 mcg per L) | |
| S3‡ | > 10 times normal | > 50,000 mIU per mL | > 10,000 ng per mL | |
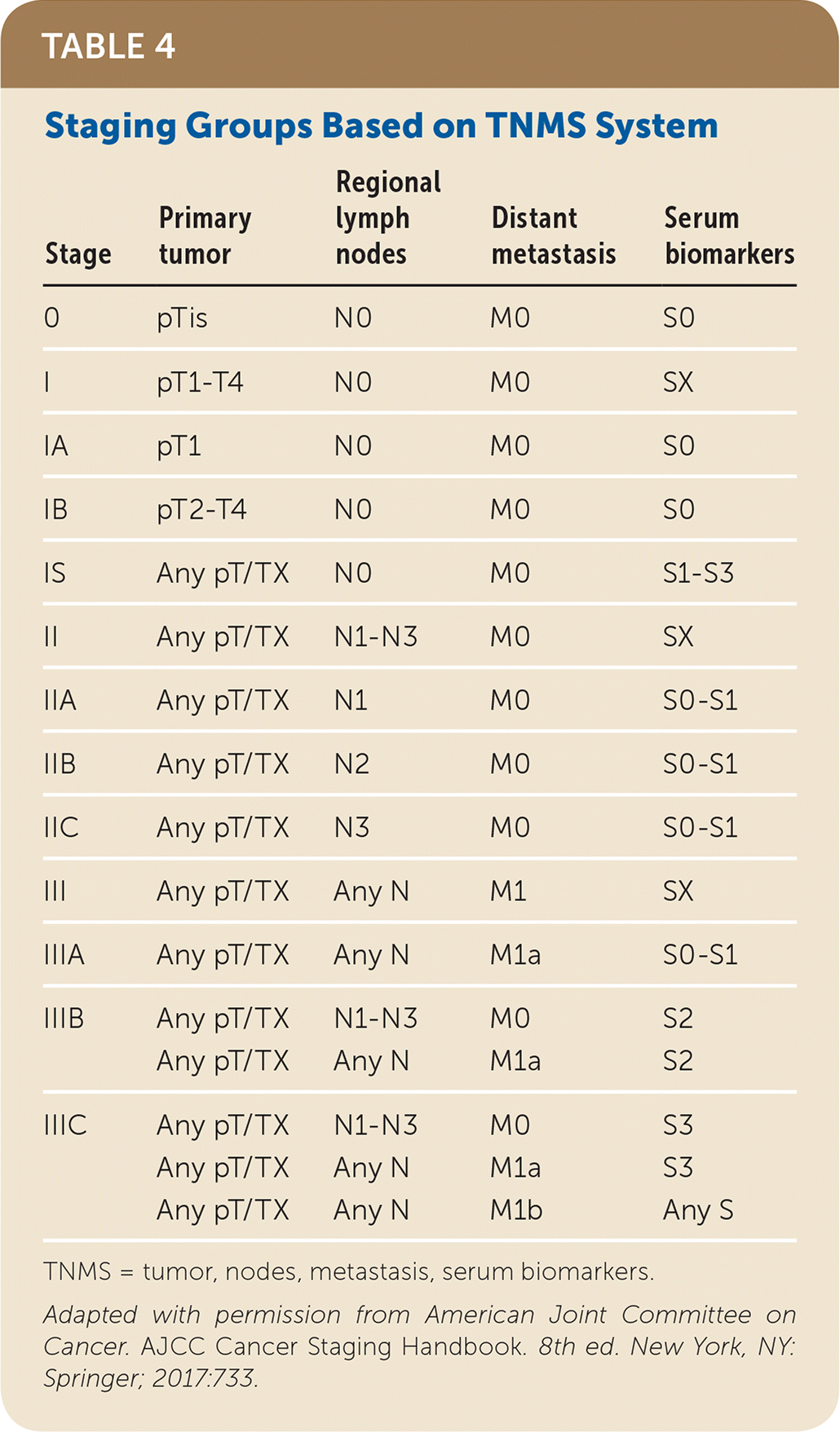
| Stage | Primary tumor | Regional lymph nodes | Distant metastasis | Serum biomarkers |
|---|---|---|---|---|
| 0 | pTis | N0 | M0 | S0 |
| I | pT1–T4 | N0 | M0 | SX |
| IA | pT1 | N0 | M0 | S0 |
| IB | pT2–T4 | N0 | M0 | S0 |
| IS | Any pT/TX | N0 | M0 | S1–S3 |
| II | Any pT/TX | N1–N3 | M0 | SX |
| IIA | Any pT/TX | N1 | M0 | S0–S1 |
| IIB | Any pT/TX | N2 | M0 | S0–S1 |
| IIC | Any pT/TX | N3 | M0 | S0–S1 |
| III | Any pT/TX | Any N | M1 | SX |
| IIIA | Any pT/TX | Any N | M1a | S0–S1 |
| IIIB | Any pT/TX | N1–N3 | M0 | S2 |
| Any pT/TX | Any N | M1a | S2 | |
| IIIC | Any pT/TX | N1–N3 | M0 | S3 |
| Any pT/TX | Any N | M1a | S3 | |
| Any pT/TX | Any N | M1b | Any S |
Treatment and Prognosis
Radical inguinal orchiectomy, including removal of the spermatic cord to the internal inguinal ring, is the primary treatment for any malignant tumor found on surgical exploration of a testicular mass. Testis-sparing surgery is generally not recommended but may be performed for a small tumor in one testis or for small bilateral tumors. Orchiectomy may be delayed if life-threatening metastases require more urgent attention.26,31
Treatment after orchiectomy is based on histology, staging, prognosis, and an individualized discussion with the patient on the benefits and harms of treatment options.24 As treatment has advanced and survival rates have improved, efforts have increasingly focused on decreasing treatment-associated morbidity through more active surveillance and selecting therapies tailored to individualized risk (i.e., risk-adapted therapy). Risk-adapted therapy is used in nonseminoma treatment but has not been validated in seminoma treatment.25,26
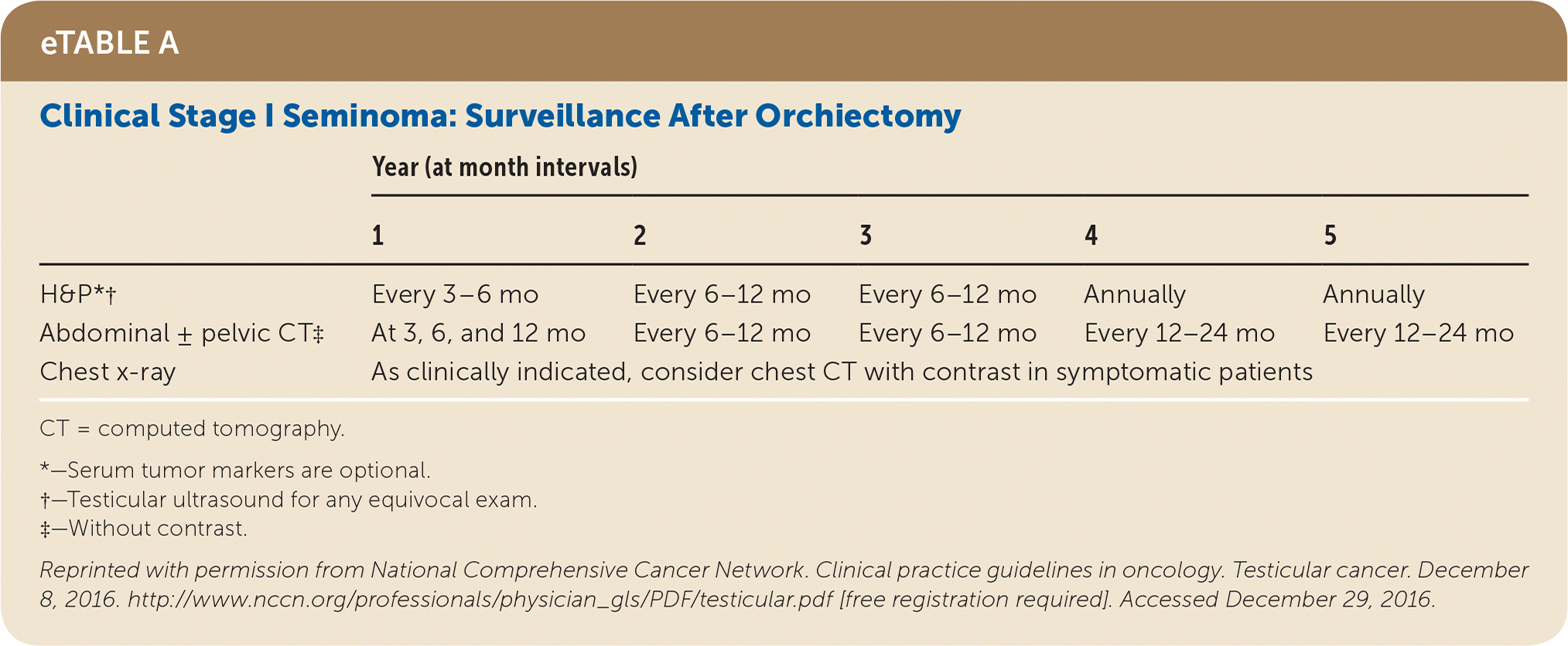
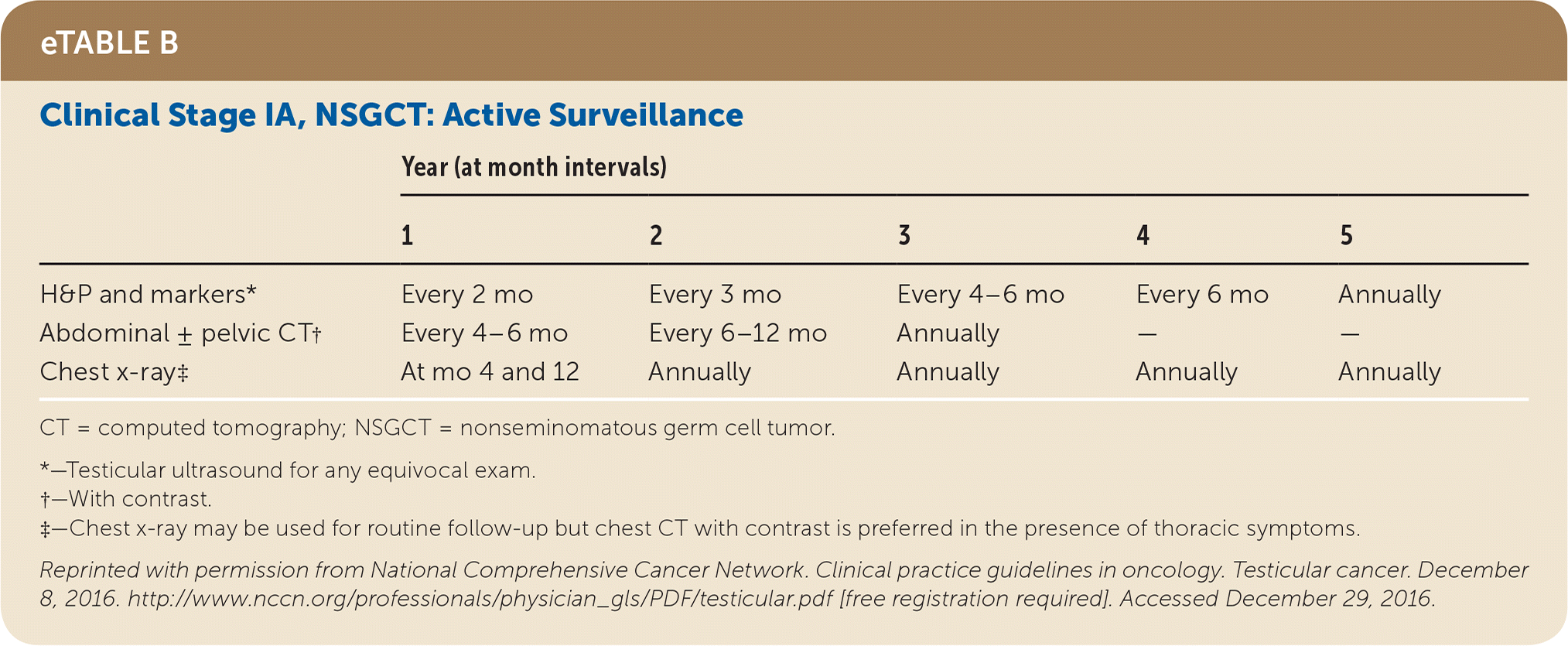
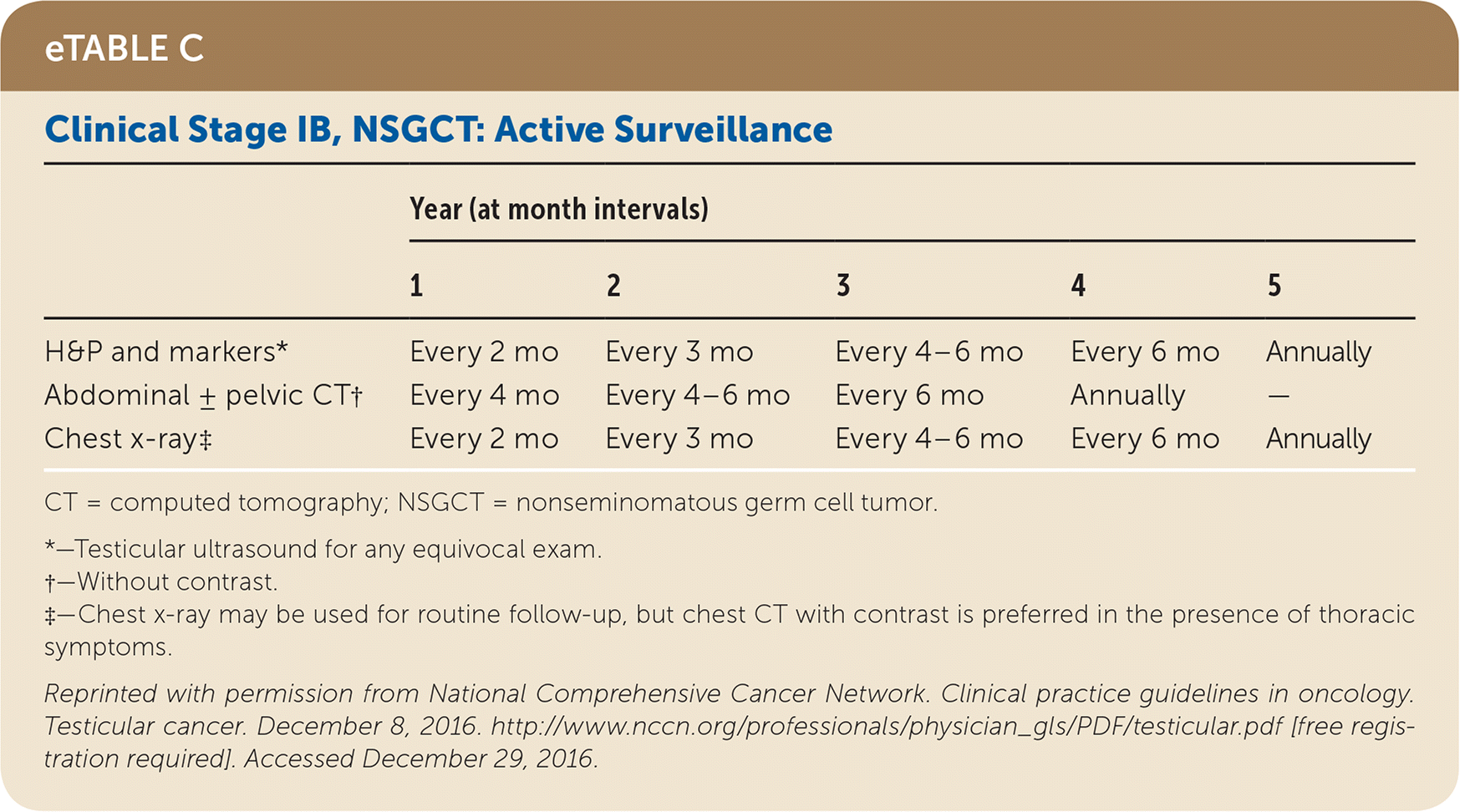
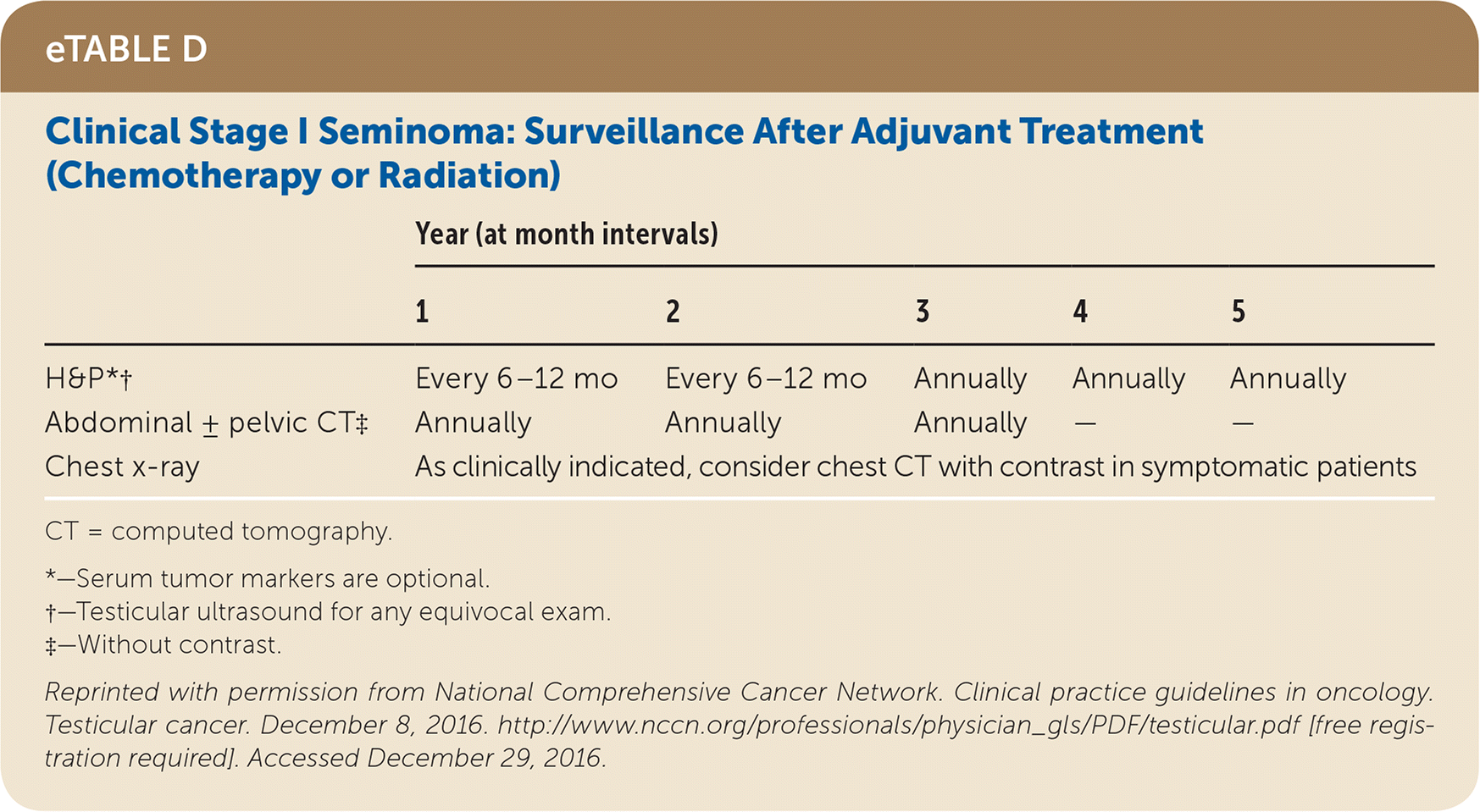
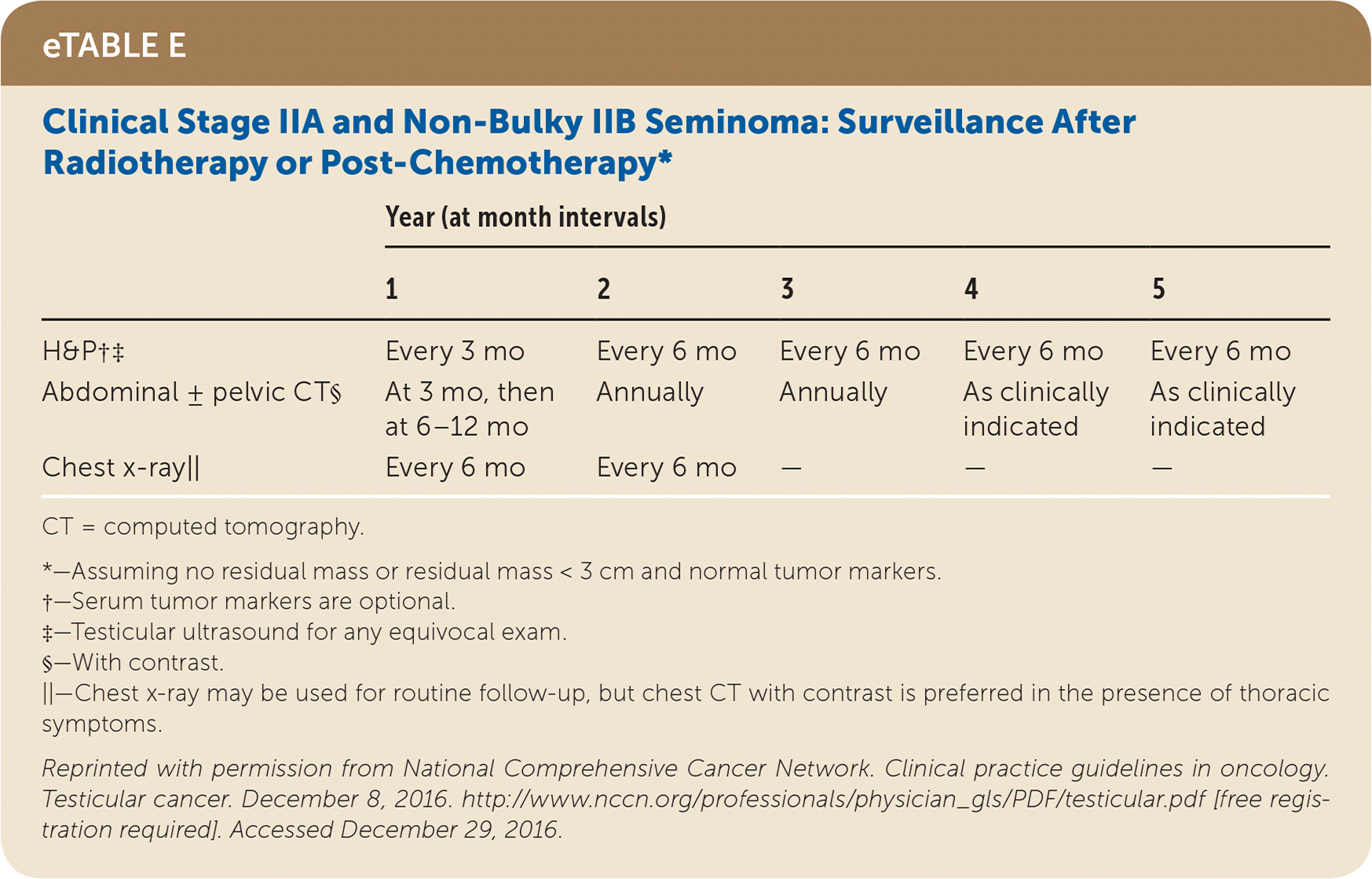
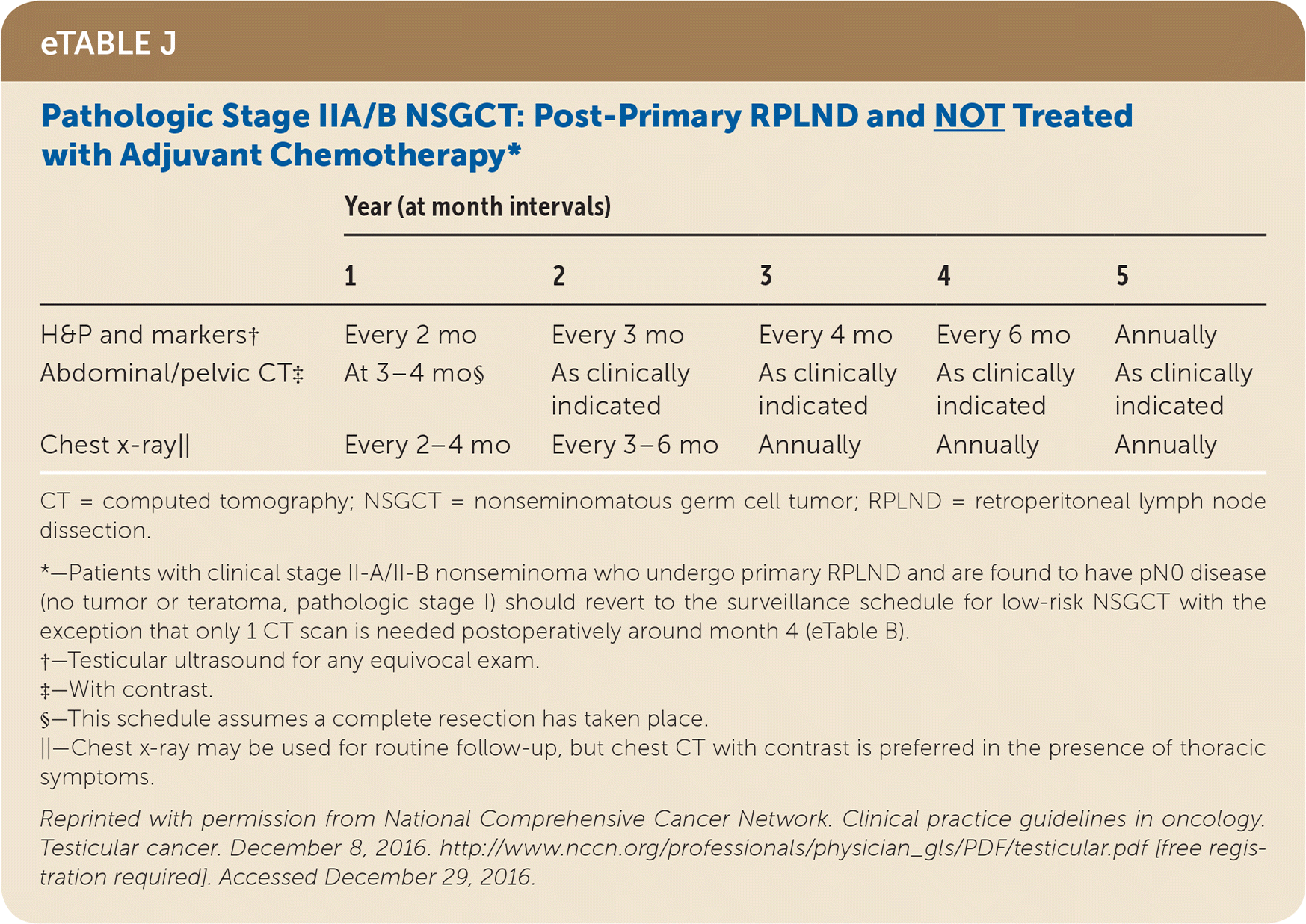
Active surveillance involves more frequent monitoring than adjuvant therapy and is associated with higher recurrence rates, but it avoids the risks of radiation and chemotherapy.24,25 The risk of testicular cancer recurrence is greatest within two to three years of primary treatment, and surveillance is continued for up to five years. This article reviews treatment (Table 525) and surveillance (eTable A, eTable B, eTable C, eTable D, eTable E, eTable F, eTable G, eTable H, eTable I, and eTable J) for germ cell tumors.

| Seminoma | Nonseminoma |
|---|---|
| Stage I | |
| pT1–pT3 tumors: Active surveillance is preferred; single-agent carboplatin or radiotherapy is an alternative | |
| Stage II | |
| |
| Stage III | |
| Chemotherapy with four cycles of EP or three cycles of BEP† | |
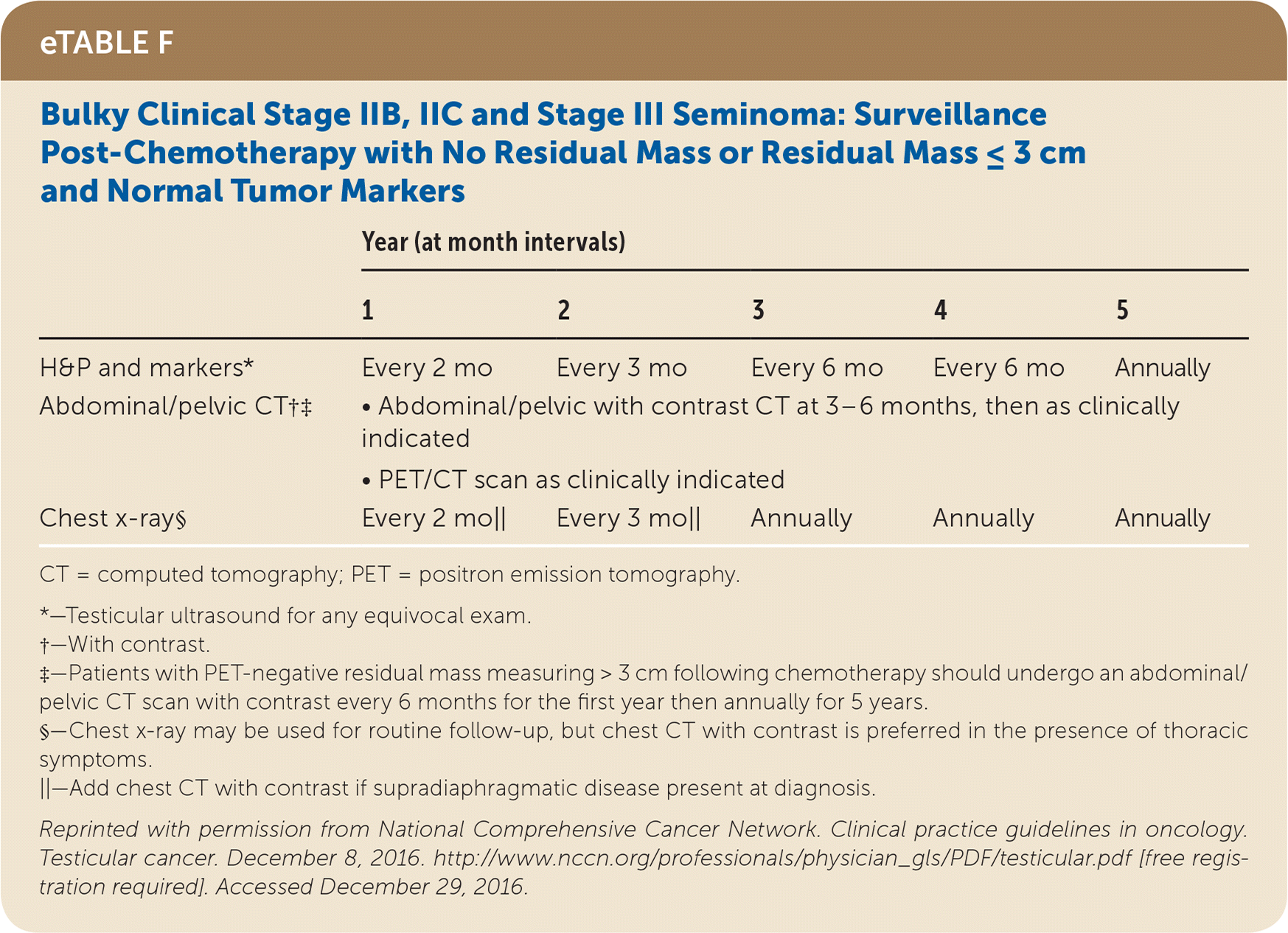
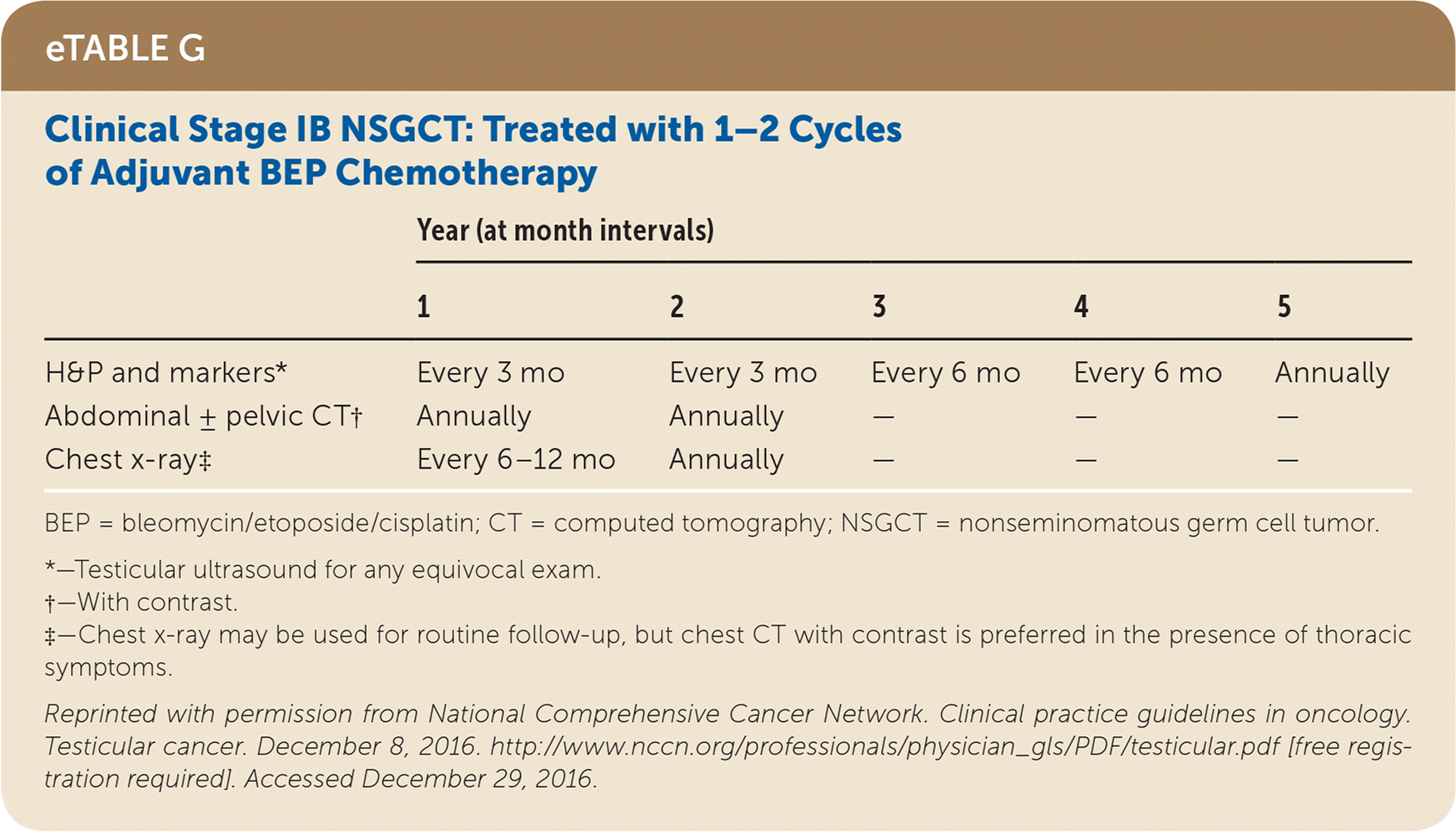
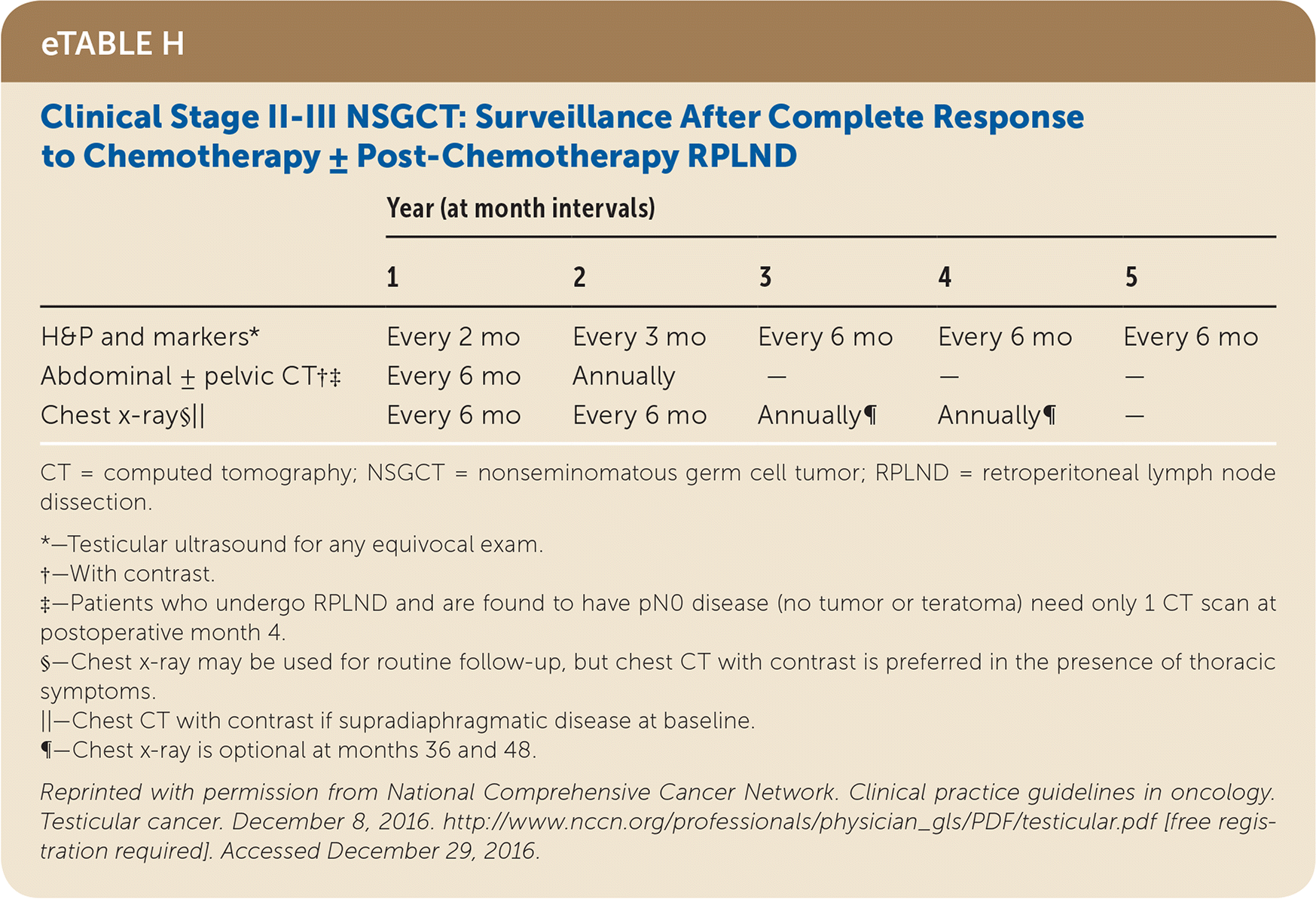
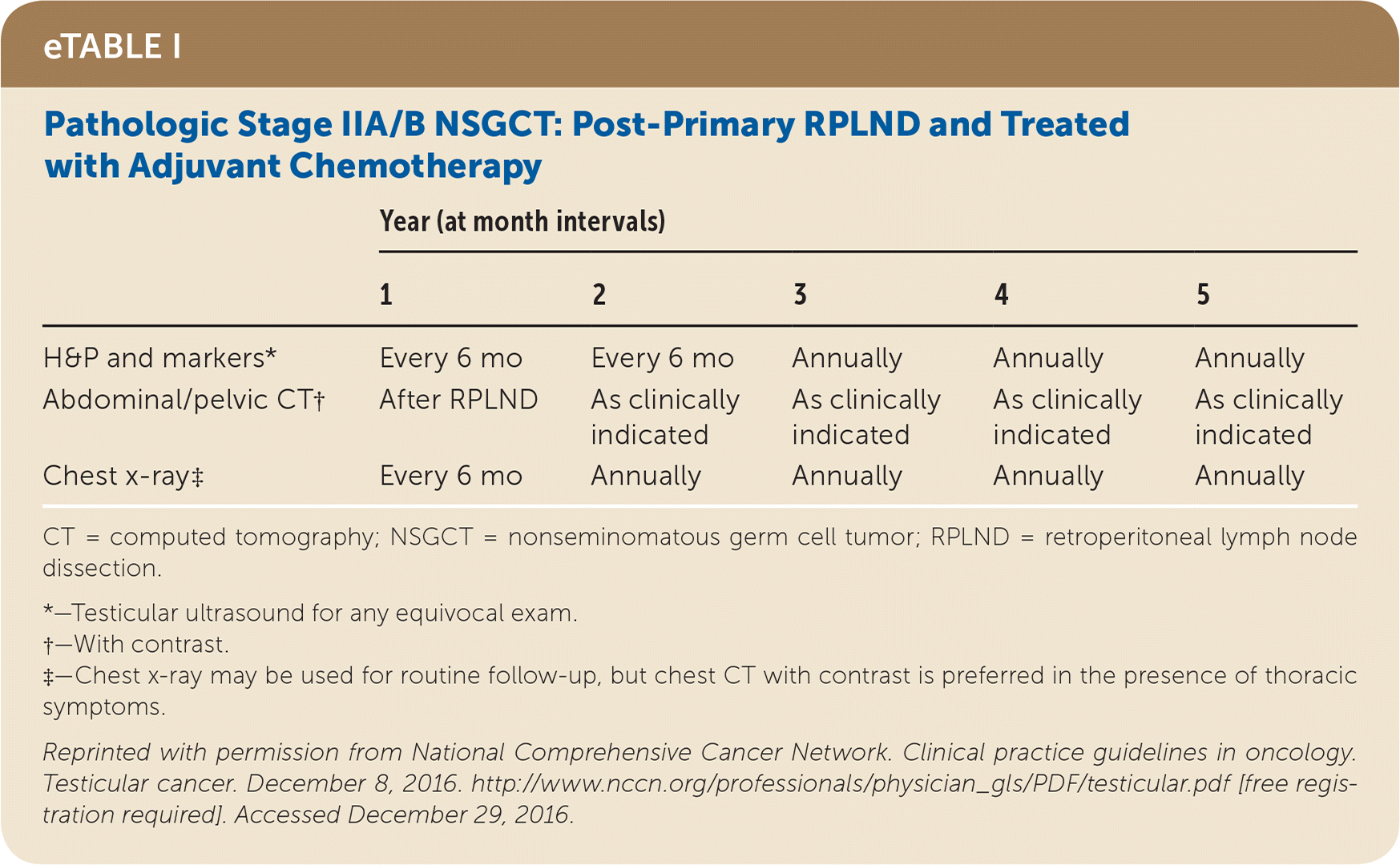
SEMINOMA TREATMENT
One-half of germ cell tumors are seminomas, and 80% to 85% are stage I at diagnosis.32 Among men with a stage I seminoma, 83% to 85% are free from relapse five years after orchiectomy alone; therefore, surveillance without additional therapy is preferred.25,33,34 Patients with risk factors for relapse (i.e., tumor invasion of the rete testis or tumor size greater than 4 cm) may be candidates for adjuvant therapy with carboplatin or radiotherapy, which further reduces the risk of relapse by 83%.32 Both adjuvant therapies yield five-year relapse-free rates near 95%, although carboplatin is considered less toxic than radiotherapy.35 The five-year survival rate after orchiectomy in patients with stage I seminoma is more than 99%, regardless of whether surveillance or adjuvant therapy is used.25,34
Stage II seminomas are treated with adjuvant therapy (radiotherapy or cisplatin-based chemotherapy, which usually includes etoposide [Toposar]). The largest meta-analysis comparing these two therapies found that they had similar relapse rates of 11% and 8% and overall survival of 98% and 99%, respectively, over a median six-year follow-up.36 Stage II and III seminomas treated with chemotherapy after orchiectomy require subsequent chest and abdominal/pelvic CT, tumor markers, and possibly positron emission tomography to determine whether the patient needs surveillance, further chemotherapy, or retroperitoneal lymph node dissection (RPLND).24,25
NONSEMINOMA TREATMENT
Nonseminomas, also called nonseminomatous germ cell tumors, are a group of histologically distinct cancers (embryonal carcinoma; yolk sac tumors; trophoblastic tumors, including choriocarcinoma; and postpubertal teratomas) that are treated similarly. Stage I nonseminoma has an excellent prognosis, with disease-specific 15-year survival of 99.1%. It can be treated with active surveillance, cisplatin-based chemotherapy, or RPLND. With active surveillance, 30% will relapse within five years, but this is lowered to 12% if men with risk factors for relapse (i.e., vascular invasion, rete testis invasion, and embryonal carcinoma) are excluded.37 Chemotherapy, which is indicated for men with risk factors for relapse, has a five-year relapse rate of 3% and is increasingly favored over RPLND because of lower relapse rates and avoidance of surgical complications.38,39 If RPLND is chosen, subsequent chemotherapy may be offered, depending on the TNMS classification.24
Postorchiectomy treatment for stage II and III nonseminomas includes cisplatin-based chemotherapy and/or RPLND, based on lymph node involvement and whether tumor markers remain elevated after orchiectomy. Chemotherapy with or without RPLND has a 96% to 97% five-year relapse-free rate for stage II nonseminoma.40,41 Resection of residual masses after chemotherapy is indicated if the mass is greater than 1 cm, but observation may be used for smaller masses. Generally, radiotherapy is not used in the treatment of nonseminomas.24
Follow-Up for Recurrence
All patients with testicular cancer must be followed closely for five years after primary treatment to monitor for recurrence. eTable A, eTable B, eTable C, eTable D, eTable E, eTable F, eTable G, eTable H, eTable I, and eTable J describe follow-up recommendations based on tumor type, stage, and selected treatment.
Follow-up includes a history and physical examination, with testicular ultrasonography for any detectable mass. Tumor markers are optional for stage I and IIA seminoma follow-up, but are recommended for advanced seminomas and all nonseminomas.25 Tumor marker findings can be negative in up to 35% of patients with nonseminoma relapse.24 Abdominal/pelvic CT should be performed at specified intervals to evaluate for recurrence, particularly in the retroperitoneum. Chest radiography may be considered if there is concern for thoracic metastasis, and surveillance chest CT should be included for anyone with thoracic metastases on original presentation.24,25
In one study, 76% of stage I seminoma relapses occurred within the first two years. Sites of recurrence included the retroperitoneum (85.7%), pelvic nodes (6.3%), spermatic cord (1.6%), mesentery (1.6%), and lung (1.6%).32 In another study, 70% of stage I nonseminoma relapses occurred within six months, with tumors located in the abdomen (59%), lungs (16%), abdomen and lungs (7%), or inguinal region (5%).37 Treatment options for relapse include chemotherapy, tumor resection, and peripheral blood stem cell transplantation.
Survivorship
The high survival rate and young age of patients with testicular cancer result in long periods of survivorship with multiple sources of potential morbidity and mortality. Complications arise from both the disease process and treatment, and vary according to individual course.
SECONDARY MALIGNANCY
Testicular cancer survivors treated with radiotherapy or chemotherapy are at increased risk of secondary malignant neoplasms. There is a threefold increased risk of leukemia among testicular cancer survivors treated with radiotherapy to the abdomen and pelvis. Cisplatin and etoposide therapies also increase the risk of secondary leukemia.42
Radiotherapy and chemotherapy increase the risk of solid cancers beginning five years after treatment.42 In a study of more than 40,000 testicular cancer survivors, the relative risk of solid cancers was 2.0 for radiotherapy alone, 1.8 for chemotherapy alone, and 2.9 for radiotherapy plus chemotherapy. Sites of secondary malignancy, in order of greatest to least risk, included stomach, pancreas, pleura, bladder, colon, and esophagus.43 Melanoma and thyroid, kidney, bladder, and connective tissue cancers are other potential secondary malignant neoplasms.42
INFERTILITY AND HYPOGONADISM
Cancer alone may affect fertility. A systematic review found that up to 50% of patients with testicular cancer have semen abnormalities prior to orchiectomy.44 Orchiectomy and the effects of chemotherapy and radiation also carry significant risks of future infertility. RPLND may induce retrograde ejaculation or anejaculation, although advances in surgical techniques have reduced this risk.45
Among testicular cancer survivors, 48% to 92% successfully have children posttreatment, often with the use of assisted reproductive techniques.46 There is wide consensus that sperm banking should be offered to patients early in the course of treatment,24–26,47 although multiple studies have demonstrated that this service often is not used.48,49 Patients should also be offered a testicular prosthesis after orchiectomy.26
Testicular cancer and its treatment increase the risk of hypogonadism.50 A multifactorial etiology is likely, including orchiectomy, chemotherapy and radiation effects, and testicular dysgenesis.44,50 Laboratory evaluation, including testosterone, luteinizing hormone, and follicle-stimulating hormone measurements, should be considered in patients with symptoms of hypogonadism. There are no specific recommendations for hypogonadism screening or treatment in testicular cancer survivors, and some studies suggest that there is no difference in quality of life between survivors with and without hypogonadism.51,52
CARDIOVASCULAR DISEASE
Adjuvant therapy increases the short- and long-term risks of cardiovascular disease in testicular cancer survivors. A recent population-based study of more than 15,000 testicular cancer survivors found a fivefold increased risk of cardiovascular mortality within the first year after chemotherapy compared with orchiectomy alone. This cardiovascular risk was not significant beyond the first year.53
A retrospective study of testicular cancer survivors compared with age-matched control patients showed that radiotherapy or chemotherapy imposes greater than a twofold increased long-term risk (after 10 years posttreatment) for atherosclerotic disease; combined therapy increased the risk to almost fivefold.54 Mediastinal irradiation, in particular, carries more risk than subphrenic irradiation.55
There are no evidence-based recommendations for heart disease screening in testicular cancer survivors. Physicians should be aware of potential cardiovascular risk in cancer survivors with attention to standard risk factor screening and lifestyle interventions for primary cardiovascular disease prevention.
OTHER COMPLICATIONS
Pulmonary toxicity may manifest as restrictive lung disease after bleomycin treatment. Nephrotoxicity, ototoxicity, and peripheral neuropathy are possible after cisplatin use.42
Data Sources: A PubMed search was completed using the following key terms: testicular cancer, germ cell tumor, seminoma, and nonseminoma. The searches included meta-analyses, randomized controlled trials, clinical trials, and reviews. We also searched the Cochrane Database of Systematic Reviews, Essential Evidence Plus, the American Cancer Society, the U.S. Preventive Services Task Force, and the National Guideline Clearinghouse. In addition, references in these resources were searched. Search dates: September 2016 through September 2017.
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of Defense, the U.S. Army Medical Corps, or the U.S. Army at large.
