Am Fam Physician. 2018;97(6):374-375
Author disclosure: No relevant financial affiliations.
Lesinurad (Zurampic) is a uricosuric agent labeled for the treatment of hyperuricemia that is associated with gout. It should be used in patients who are already taking appropriate doses of a xanthine oxidase inhibitor such as allopurinol, but who have not yet reached target levels of serum uric acid.1
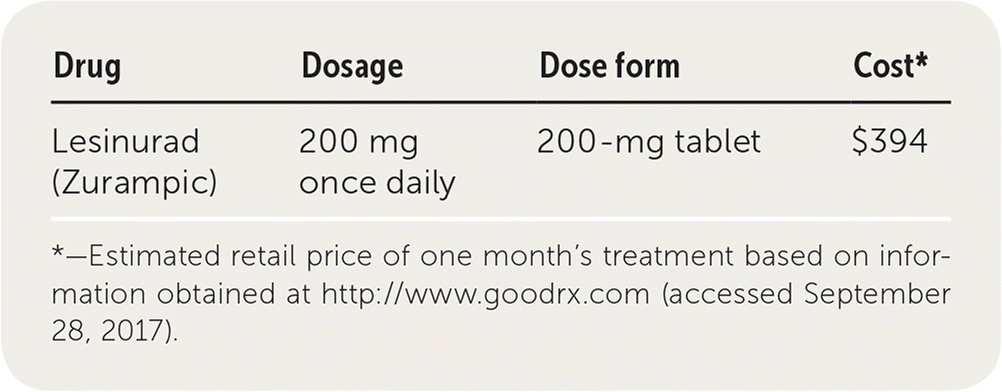
| Drug | Dosage | Dose form | Cost* |
|---|---|---|---|
| Lesinurad (Zurampic) | 200 mg once daily | 200-mg tablet | $394 |
Safety
Lesinurad can cause acute renal failure if taken without a xanthine oxidase inhibitor, at a higher dose than recommended, or by patients with moderately reduced renal function (creatinine clearance less than 45 mL per minute per 1.73 m2 [0.75 mL per second per m2]).1 Renal function should be monitored in all patients, especially those with a creatinine clearance less than 60 mL per minute per 1.73 m2 (1.00 mL per second per m2) or who have increased levels of serum creatinine. Lesinurad should be discontinued in patients with symptoms of uric acid nephropathy or serum creatinine elevations 1.5 to 2 times the pretreatment value.1 In premarketing research, major adverse cardiovascular events occurred at a slightly higher rate in patients taking lesinurad vs. those taking placebo, especially at higher-than-recommended doses.1
Lesinurad is metabolized in the liver by the cytochrome P450 (CYP450) 2C9 enzyme system. It may affect the blood levels of other medications metabolized by this system, and it may be affected by these other medications, although the degree to which these interactions are clinically relevant is unknown. Lesinurad may reduce the reliability of oral, injectable, transdermal, and implantable hormonal contraceptives; therefore, women taking lesinurad should use additional methods of contraception. Daily dosages of aspirin greater than 325 mg may increase uric acid levels, reducing the effect of uric acid treatment.1 Safety has not been established in pregnant or breastfeeding women.1
Tolerability
Lesinurad used in combination with a xanthine oxidase inhibitor is well tolerated. In clinical trials, the most common adverse effects (i.e., affecting more than 2% of patients) were headache, influenza, and gastroesophageal reflux disease.1,2 Approximately 3% to 8% of patients discontinue therapy because of adverse effects.3,4
Effectiveness
Adding lesinurad therapy has been shown to decrease serum uric acid levels in patients with an average uric acid concentration of 7.0 mg per dL (416 μmol per L) who are already taking at least 300 mg per day of allopurinol. Approximately one-half (55.4%) of patients will achieve uric acid levels of less than 6.0 mg per dL (357 μmol per L; number needed to treat for six months = 3; 95% confidence interval, 2.5 to 4.7).3 However, over one year, lesinurad will not reduce the likelihood of a gout flare-up or improve tophus resolution in patients with six or fewer episodes per year. It has not been studied in patients with more frequent episodes.3,4 It has also not been studied to determine its effect on the rate of uric acid nephropathy, and it has not been compared with other uricosuric agents added to allopurinol, such as probenecid.
Price
A one-month supply of lesinurad costs approximately $394. It is significantly more expensive than probenecid, which costs about $34 for a 30-day supply. In addition, a one-month supply of allopurinol costs about $10.
Simplicity
Lesinurad in a dosage of 200 mg per day should be taken with food and water at the same time as the morning dose of the xanthine oxidase inhibitor (at least 300 mg of allopurinol or an equivalent). Prophylaxis for mobilization gout, such as treatment with colchicine (Colcrys, Mitigare), should be started at the beginning of therapy. Patients should stop taking lesinurad if the xanthine oxidase inhibitor is discontinued.
Bottom Line
Lesinurad taken in addition to a xanthine oxidase inhibitor reduces serum uric acid levels. However, it does not reduce the likelihood of experiencing a gout flare-up or completely resolving tophi. Although lesinurad may be useful as an add-on therapy for patients who require once-daily dosing of uricosuric agents, it is much more expensive than current therapy.