Am Fam Physician. 2018;97(6):385-393
Patient information: A handout on pancreatitis is available.
Author disclosure: No relevant financial affiliations.
Chronic pancreatitis is an irreversible and progressive disorder of the pancreas characterized by inflammation, fibrosis, and scarring. Exocrine and endocrine functions are lost, often leading to chronic pain. The etiology is multifactorial, although alcoholism is the most significant risk factor in adults. The average age at diagnosis is 35 to 55 years. If chronic pancreatitis is suspected, contrast-enhanced computed tomography is the best imaging modality for diagnosis. Computed tomography may be inconclusive in early stages of the disease, so other modalities such as magnetic resonance imaging, magnetic resonance cholangiopancreatography, or endoscopic ultrasonography with or without biopsy may be used. Recommended lifestyle modifications include cessation of alcohol and tobacco use and eating small, frequent, low-fat meals. Although narcotics and antidepressants provide the most pain relief, one-half of patients eventually require surgery. Therapeutic endoscopy is indicated to treat symptomatic strictures, stones, and pseudocysts. Decompressive surgical procedures, such as lateral pancreaticojejunostomy, are indicated for large duct disease (pancreatic ductal dilation of 7 mm or more). Resection procedures, such as the Whipple procedure, are indicated for small duct disease or pancreatic head enlargement. The risk of pancreatic cancer is increased in patients with chronic pancreatitis, especially hereditary pancreatitis. Although it is not known if screening improves outcomes, clinicians should counsel patients on this increased risk and evaluate patients with weight loss or jaundice for neoplasm.
Chronic pancreatitis is a permanent, progressive destruction of pancreatic tissue and function. Clinical manifestations include disabling abdominal pain, steatorrhea, and diabetes mellitus.1–3 Incidence and prevalence remain low and, despite advances in medical imaging, definitive diagnosis remains challenging.3 Primary care physicians should be familiar with presenting clinical features, as well as treatment options and long-term complications.
WHAT IS NEW ON THIS TOPIC
A meta-analysis of 43 studies that included more than 3,400 patients concluded that computed tomography, magnetic resonance imaging, endoscopic retrograde cholangiopancreatography, and endoscopic ultrasonography have comparably high diagnostic accuracy for chronic pancreatitis; therefore, a stepwise approach based on cost, invasiveness, and availability is recommended.
A Cochrane review of 10 trials involving 361 participants suggested that pancreatic enzyme replacement does not reduce pain from chronic pancreatitis.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Contrast-enhanced computed tomography is the recommended initial imaging study in patients with suspected chronic pancreatitis. | C | 3, 10, 22–24 |
| Endoscopic ultrasonography is favored over endoscopic retrograde cholangiopancreatography for the endoscopic diagnosis of chronic pancreatitis because of its increased safety and ability to evaluate the pancreatic parenchyma and duct system. | C | 3 |
| Antioxidant therapy does not improve pain control or mortality outcomes in patients with chronic pancreatitis. | B | 10, 35 |
| Pancreatic enzyme replacement is indicated for steatorrhea and malabsorption and may help relieve pain in patients with chronic pancreatitis. | B | 38–42 |
| Endoscopic drainage of pseudocysts results in a similar rate of pain relief as surgery, with equivalent or lower mortality. | B | 25, 44–47, 53, 54 |
| Pancreatoduodenectomy (Whipple procedure, pylorus-preserving, or duodenum-preserving) is indicated in the treatment of chronic pancreatitis with pancreatic head enlargement and typically results in significant pain relief. | B | 8, 49, 55 |
Etiology
Data from case series and cross-sectional studies estimate that the incidence of chronic pancreatitis is between about four and 12 per 100,000 persons per year. Data on prevalence are scarce, with estimates ranging from 37 to 42 per 100,000 persons.3 Men are affected 1.5 to 3 times more than women.4 The average age at diagnosis is 35 to 55 years. Alcoholism is the most significant risk factor for the development of chronic pancreatitis, accounting for 70% of cases in adults. Genetic disease, especially cystic fibrosis, and anatomic abnormalities are the most common causes in children. Table 1 includes the TIGAR-O (toxic-metabolic, idiopathic, genetic, autoimmune, recurrent and severe acute pancreatitis, obstructive) classification of common risk factors for chronic pancreatitis.4,5

| Toxic-metabolic |
| Alcohol |
| Chronic renal failure |
| Hypercalcemia (hyperparathyroidism) |
| Hyperlipidemia (rare) |
| Medications* |
| Tobacco |
| Toxins |
| Idiopathic |
| Early and late onset |
| Tropical pancreatitis (tropical calcifying pancreatitis and fibrocalculous pancreatic diabetes) |
| Genetic |
| Autosomal dominant (cationic trypsinogen [codon 29 and 122 mutations]) |
| Autosomal recessive modifier genes (CFTR and SPINK1 mutations, cationic trypsinogen [codon 16, 22, and 23 mutations], alpha1-antitrypsin deficiency) |
| Autoimmune |
| Autoimmune chronic pancreatitis associated with inflammatory bowel disease, Sjögren syndrome, primary biliary cirrhosis |
| Isolated autoimmune chronic pancreatitis |
| Recurrent and severe acute pancreatitis |
| Postirradiation |
| Postnecrotic (severe acute pancreatitis) |
| Recurrent acute pancreatitis |
| Vascular ischemia |
| Obstructive |
| Duct obstruction (pancreatic or ampullary tumors) posttraumatic pancreatic duct fibrosis) |
| Pancreas divisum |
| Sphincter of Oddi disorders |
Pathophysiology
The pathophysiology of chronic pancreatitis involves progressive fibrotic destruction of the pancreas in response to inflammation. A simple model involving the “two-hit” theory has been proposed. The first hit is acute pancreatitis, causing injury to the pancreas. The second hit is an abnormal inflammatory response to this injury. This causes sustained activation of pancreatic profibrotic cells, including stellate cells. In at-risk individuals, this can result in collagen deposition and fibrosis, which can lead to chronic pancreatitis.2–4,6–8 However, a subset of chronic pancreatitis is caused by autoimmune and genetic factors. Chronic pancreatitis is autoimmune in 5% to 6% of cases.9 Autoimmune pancreatitis has a distinctive histologic appearance with lymphocytic infiltration, but also results in fibrosis and loss of pancreatic function.3
Diagnosis of Chronic Pancreatitis
CLINICAL PRESENTATION
Patients most commonly present with recurrent episodes of acute pancreatitis. This will often progress to chronic abdominal pain that is characteristically located in the epigastrium and radiates to the back. The pain is worsened by eating and relieved by leaning forward. In time, it may spontaneously resolve as the pancreas fails.2,10–12 Although most patients present with pain, pancreatitis is painless in roughly 10% to 20% of patients.1 When about 90% of pancreatic function is lost, patients will have signs of exocrine dysfunction, such as steatorrhea, malabsorption, and fat-soluble vitamin deficiencies (A, D, E, K, B12).1,10,13,14 Endocrine dysfunction, such as diabetes, will also develop.1,13
DIFFERENTIAL DIAGNOSIS
Table 2 includes a comprehensive differential diagnosis.15 Amylase and lipase levels may be elevated in patients with chronic pancreatitis, but these elevations may occur with other conditions that also present with abdominal pain, such as mesenteric ischemia, biliary disease, renal disease, and complicated peptic ulcer disease.2,13,16–18
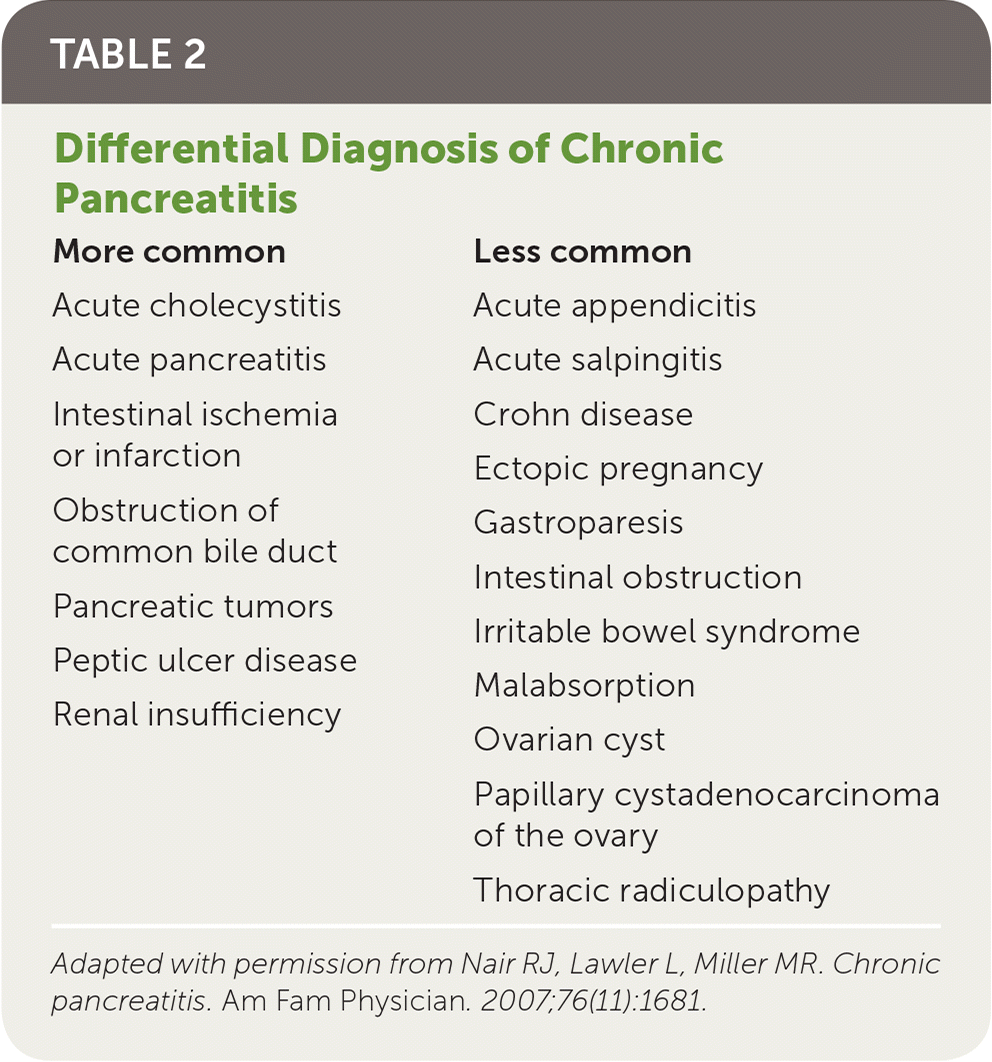
| More common | Less common |
|---|---|
| Acute cholecystitis | Acute appendicitis |
| Acute pancreatitis | Acute salpingitis |
| Intestinal ischemia or infarction | Crohn disease |
| Obstruction of common bile duct | Ectopic pregnancy |
| Pancreatic tumors | Gastroparesis |
| Peptic ulcer disease | Intestinal obstruction |
| Renal insufficiency | Irritable bowel syndrome |
| Malabsorption | |
| Ovarian cyst | |
| Papillary cystadenocarcinoma of the ovary | |
| Thoracic radiculopathy |
DIAGNOSTIC TESTING
The diagnosis of chronic pancreatitis is made based on a patient's history, clinical presentation, and imaging findings. There is no single test that is diagnostic of chronic pancreatitis, especially in the early stages. If chronic pancreatitis is suspected based on history and physical examination, a diagnostic workup should be initiated. Diagnostic tests should be chosen based on their availability after consideration of risks and benefits.1,2,4,9,18–20 Laboratory tests used in the evaluation of patients with suspected chronic pancreatitis are summarized in Table 3.15
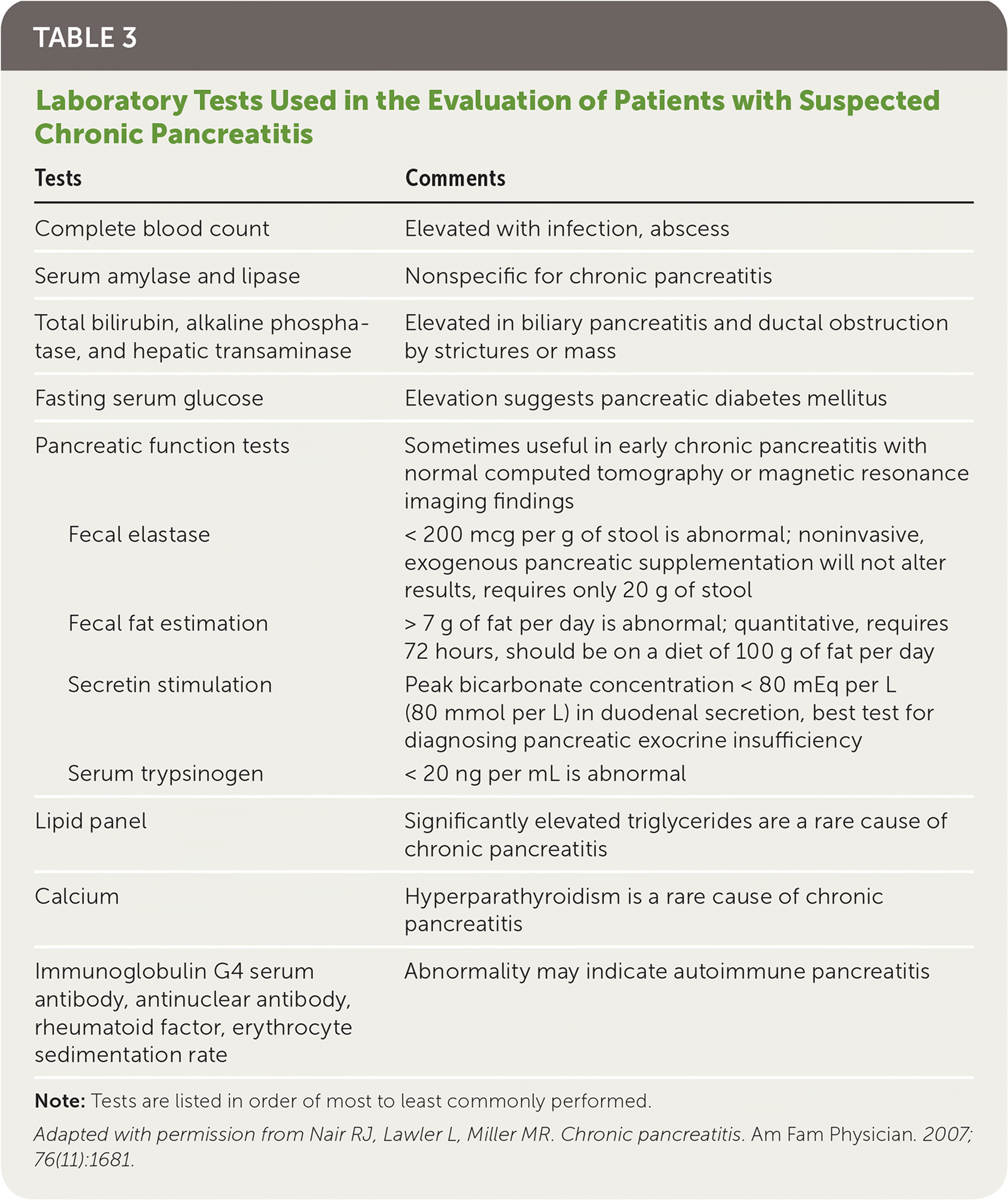
| Tests | Comments | |
|---|---|---|
| Complete blood count | Elevated with infection, abscess | |
| Serum amylase and lipase | Nonspecific for chronic pancreatitis | |
| Total bilirubin, alkaline phosphatase, and hepatic transaminase | Elevated in biliary pancreatitis and ductal obstruction by strictures or mass | |
| Fasting serum glucose | Elevation suggests pancreatic diabetes mellitus | |
| Pancreatic function tests | Sometimes useful in early chronic pancreatitis with normal computed tomography or magnetic resonance imaging findings | |
| Fecal elastase | < 200 mcg per g of stool is abnormal; noninvasive, exogenous pancreatic supplementation will not alter results, requires only 20 g of stool | |
| Fecal fat estimation | > 7 g of fat per day is abnormal; quantitative, requires 72 hours, should be on a diet of 100 g of fat per day | |
| Secretin stimulation | Peak bicarbonate concentration < 80 mEq per L (80 mmol per L) in duodenal secretion, best test for diagnosing pancreatic exocrine insufficiency | |
| Serum trypsinogen | < 20 ng per mL is abnormal | |
| Lipid panel | Significantly elevated triglycerides are a rare cause of chronic pancreatitis | |
| Calcium | Hyperparathyroidism is a rare cause of chronic pancreatitis | |
| Immunoglobulin G4 serum antibody, antinuclear antibody, rheumatoid factor, erythrocyte sedimentation rate | Abnormality may indicate autoimmune pancreatitis | |
Laboratory Testing. In acute pancreatitis, pancreatic enzymes are elevated more than three times the upper limit of normal.16,18 In chronic pancreatitis, these enzymes may be only mildly elevated or normal, especially as the pancreas is replaced by increasing amounts of fibrotic tissue later in the disease process.1 If the biliary tract is obstructed (5% to 10% of cases), bilirubin, alkaline phosphatase, and hepatic transaminases may be elevated.1,16
Pancreatic function tests use a catheter inserted into the pancreatic duct to directly measure pancreatic enzyme secretion. These tests are expensive and challenging to perform. The positive predictive value of these tests is just 45%, but the negative predictive value is 97%. Therefore, although they are not recommended as part of the routine workup, they can be useful for ruling out chronic pancreatitis in patients who exhibit clinical features of pancreatitis but have normal imaging.1,2,4,10,19–21 Fecal elastase is an indirect measure of pancreatic exocrine function that has high sensitivity (65% to 100%) and poor specificity (55%). The high false-positive rate makes this a poor screening test, but it can be helpful in ruling out pancreatitis.19
Imaging Studies. The radiologic evaluation of a patient with suspected chronic pancreatitis should progress from least invasive to more invasive. A meta-analysis of 43 studies that included more than 3,400 patients concluded that computed tomography (CT), magnetic resonance imaging (MRI), endoscopic retrograde cholangiopancreatography, and endoscopic ultrasonography (EUS) have comparably high diagnostic accuracy; therefore, a stepwise approach based on cost, invasiveness, and availability is recommended.22 eTable A includes the sensitivity and specificity of available imaging techniques. Contrast-enhanced CT of the pancreas is the first-choice modality because it is noninvasive and readily available.3 Ductal calcifications are pathognomonic findings of chronic pancreatitis (Figure 115). CT can detect conditions that mimic chronic pancreatitis, as well as complications of chronic pancreatitis, such as pseudocysts, ductal dilation, pseudoaneurysms, necrosis, and parenchymal atrophy.3,10,22–24
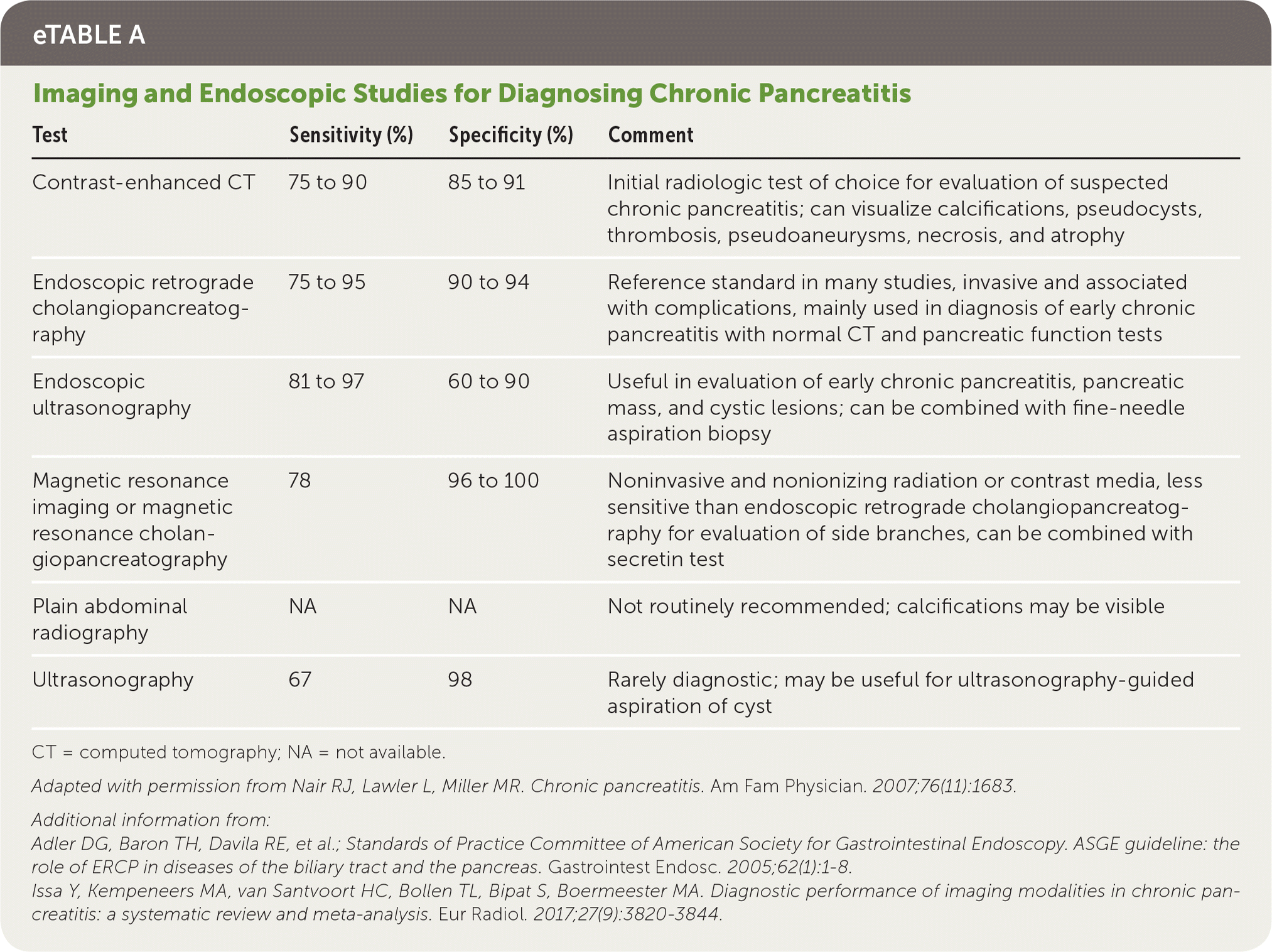
| Test | Sensitivity (%) | Specificity (%) | Comment |
|---|---|---|---|
| Contrast-enhanced CT | 75 to 90 | 85 to 91 | Initial radiologic test of choice for evaluation of suspected chronic pancreatitis; can visualize calcifications, pseudocysts, thrombosis, pseudoaneurysms, necrosis, and atrophy |
| Endoscopic retrograde cholangiopancreatography | 75 to 95 | 90 to 94 | Reference standard in many studies, invasive and associated with complications, mainly used in diagnosis of early chronic pancreatitis with normal CT and pancreatic function tests |
| Endoscopic ultrasonography | 81 to 97 | 60 to 90 | Useful in evaluation of early chronic pancreatitis, pancreatic mass, and cystic lesions; can be combined with fine-needle aspiration biopsy |
| Magnetic resonance imaging or magnetic resonance cholangiopancreatography | 78 | 96 to 100 | Noninvasive and nonionizing radiation or contrast media, less sensitive than endoscopic retrograde cholangiopancreatography for evaluation of side branches, can be combined with secretin test |
| Plain abdominal radiography | NA | NA | Not routinely recommended; calcifications may be visible |
| Ultrasonography | 67 | 98 | Rarely diagnostic; may be useful for ultrasonography-guided aspiration of cyst |
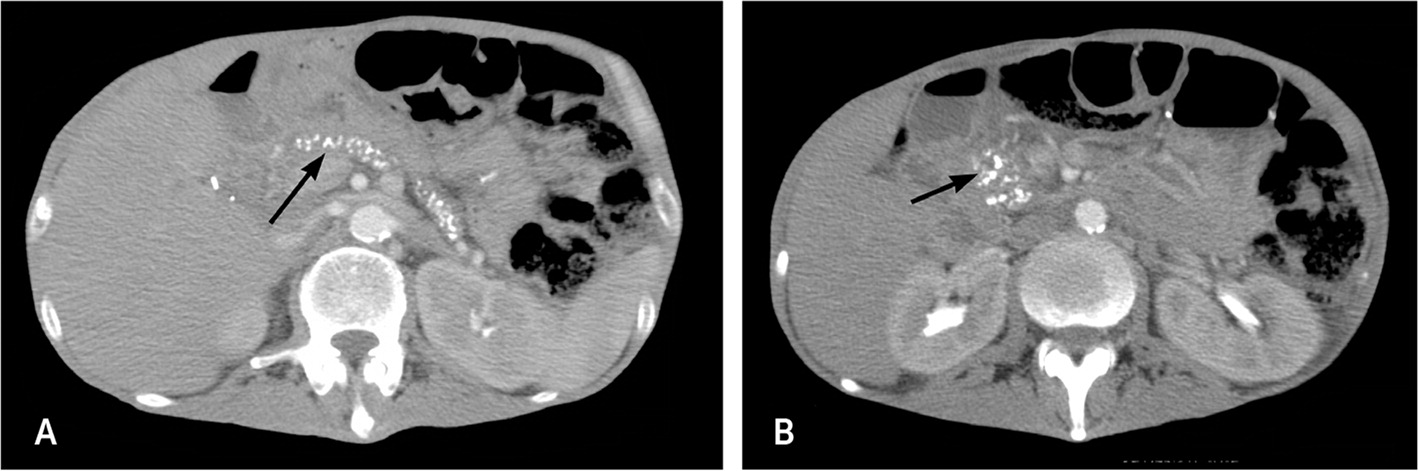
If CT findings are equivocal, patients may require referral for more focused pancreatic imaging, such as MRI or magnetic resonance cholangiopancreatography, or for endoscopic procedures, such as EUS or endoscopic retrograde cholangiopancreatography.3 Magnetic resonance cholangiopancreatography and EUS are useful for evaluating the pancreatic parenchyma and duct system. Endoscopic retrograde cholangiopancreatography has a high risk of complications (e.g., pancreatitis, hemorrhage, infection), so it is not recommended for diagnosis unless all other tests are inconclusive.2,25
APPROACH TO A PATIENT WITH SUSPECTED CHRONIC PANCREATITIS
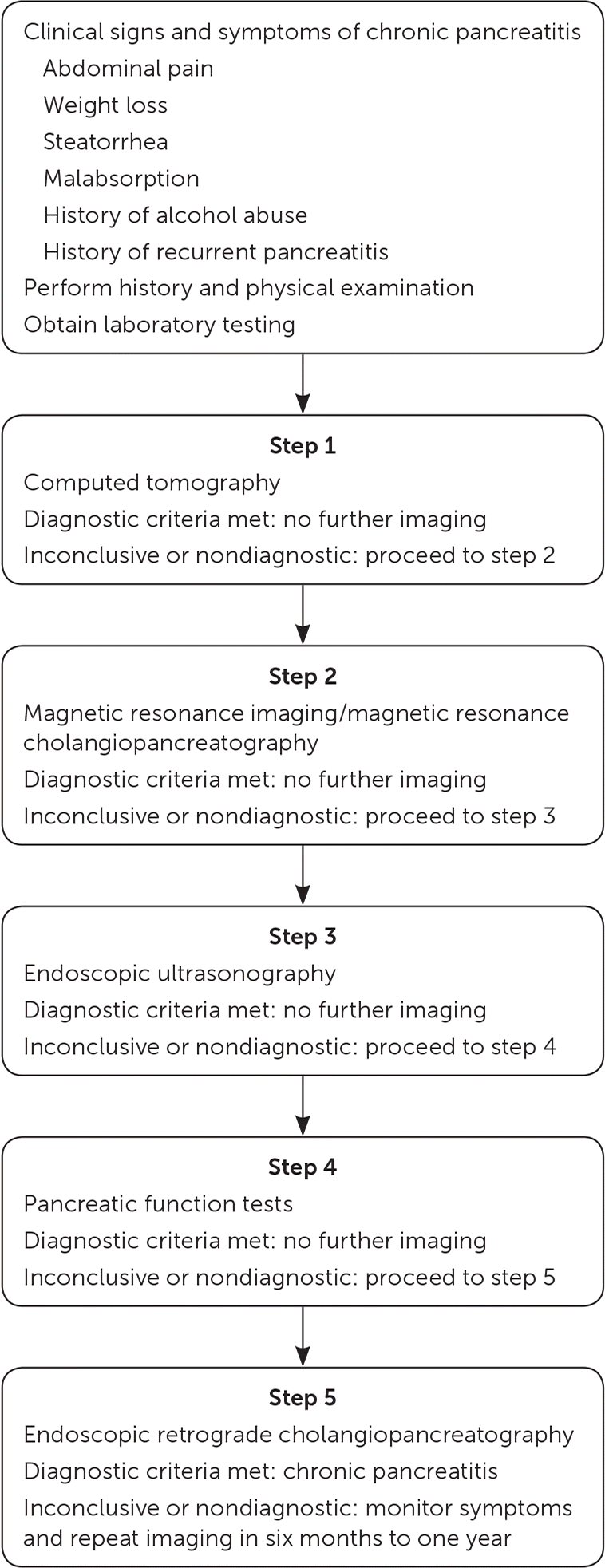
Figure 2 shows a five-step diagnostic approach to patients with signs and symptoms of chronic pancreatitis. CT will detect involvement of large duct disease (pancreatic ductal dilation of 7 mm or more).2,4,10 CT may be inconclusive in patients with early disease or small duct disease (pancreatic ductal dilation less than 7 mm). These patients should be referred to a center with expertise in pancreatic care for an MRI or magnetic resonance cholangiopancreatography.3 These imaging modalities, with EUS, are also useful for preoperative planning in patients with stones, strictures, or pseudocysts.2–4,23,25–27 If a cystic lesion or mass is identified on imaging, malignancy must be excluded through EUS with biopsy and tumor marker analysis, specifically cancer antigen 19-9.2,3,28,29 If chronic pancreatitis is still suspected despite normal imaging findings, pancreatic function tests can be performed.2–4,27–29 A small study of 25 patients suggests that combining EUS and pancreatic function tests can improve the sensitivity of diagnosis.30
Treatment
Treatment of chronic pancreatitis can be medical, endoscopic, or surgical (Table 4).15 The goals of all treatment modalities are improved pain, improved quality of life, and decreased morbidity and mortality from endocrine and exocrine pancreatic dysfunction. Studies that compare conservative medical therapy with invasive endoscopic or surgical interventions are lacking. Therefore, treatment decisions should be based on careful consideration of the goals of treatment and the risks vs. benefits.31,32

| Medical | |
| Analgesics (stepwise approach) | |
| Antidepressants (treatment of concurrent depression) | |
| Cessation of alcohol and tobacco use | |
| Denervation (celiac nerve blocks, transthoracic splanchnicectomy) | |
| Insulin (for pancreatic diabetes) | |
| Low-fat diet and small meals | |
| Pancreatic enzymes with proton pump inhibitors or histamine H2 blockers | |
| Steroid therapy (in autoimmune pancreatitis) | |
| Vitamin supplementation (A, D, E, K, and B12) | |
| Endoscopic | |
| Extracorporeal shock wave lithotripsy with or without endoscopy | |
| Pancreatic sphincterotomy and stent placement for pain relief | |
| Transampullary or transgastric drainage of pseudocyst | |
| Surgical | |
| Decompression | |
| Cystenterostomy | |
| Lateral pancreaticojejunostomy (most common) | |
| Sphincterotomy or sphincteroplasty | |
| Resection | |
| Distal or total pancreatectomy | |
| Pancreatoduodenectomy (Whipple procedure, pylorus-preserving, duodenum-preserving) | |
| Not recommended | |
| Allopurinol | |
| Antioxidant therapy (vitamin C, vitamin E, selenium, methionine [no longer available in the United States]) | |
| Octreotide (Sandostatin) | |
| Prokinetic agents (erythromycin) | |
MEDICAL
Disabling pain is the most common symptom of chronic pancreatitis. Because treatment with narcotic pain medications can lead to opioid addiction, beginning with nonsteroidal anti-inflammatory drugs or acetaminophen is recommended. Adding narcotic pain medications in a stepwise approach may be necessary to obtain adequate pain control. Adjunctive therapy with tricyclic antidepressants, selective serotonin reuptake inhibitors, and combined serotonin and norepinephrine reuptake inhibitors is used to act in synergy with narcotics and treat concomitant depression that is common in patients with chronic pancreatitis.31,32
A Cochrane review of antioxidant therapy, including selenium, beta carotene, l-methionine, vitamin C, and vitamin E, did not find evidence of effectiveness.35 Other studies found that octreotide (Sandostatin), allopurinol, or vitamin D provided no pain relief.10 In a three-week long study funded by the manufacturer, pregabalin (Lyrica) decreased short-term opioid use and improved short-term pain scores.36,37 However, studies with longer follow-up and larger sample size are needed before this becomes a standard treatment for chronic pancreatitis pain.
Pancreatic enzyme replacement is beneficial for the treatment of steatorrhea and malabsorption.38–41 A Cochrane review of 10 trials involving 361 participants suggested that pancreatic enzyme replacement does not reduce pain from chronic pancreatitis, although results of individual studies were mixed.42
ENDOSCOPY
Endoscopy can be used to treat symptomatic strictures, stones, and pseudocysts. Endoscopic drainage of pseudocysts has a similar rate of pain relief as surgery, with equivalent or lower mortality.25,43–48 Regression of the cyst occurs in 70% to 86% of patients after endoscopic drainage.25–28,43–46 Endoscopic sphincterotomy or stent placement can provide pain relief without the risks of surgery in patients with strictures.10,25,27 Extracorporeal shock wave lithotripsy with or without endoscopic drainage of the pancreatic duct can reduce stone burden and pain.10,25,49,50
SURGERY
One-half of patients with chronic pancreatitis will eventually require surgery,1 most commonly because of intractable, disabling pain.51 Indications for surgery are listed in Table 5.15 There are two types of surgeries for chronic pancreatitis. Decompression procedures are performed on patients with large duct disease, whereas resection procedures are performed on patients with small duct disease or pancreatic head enlargement.
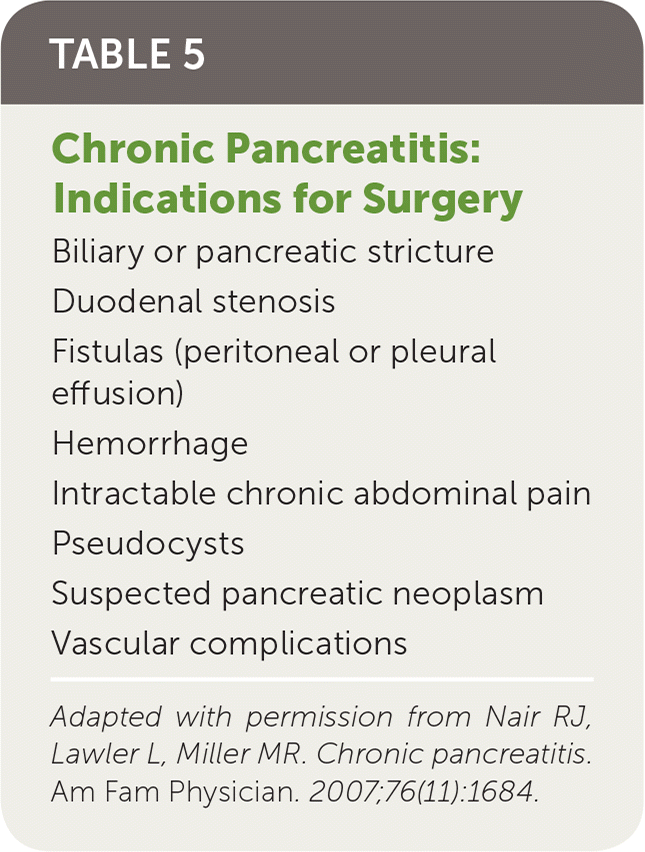
| Biliary or pancreatic stricture |
| Duodenal stenosis |
| Fistulas (peritoneal or pleural effusion) |
| Hemorrhage |
| Intractable chronic abdominal pain |
| Pseudocysts |
| Suspected pancreatic neoplasm |
| Vascular complications |
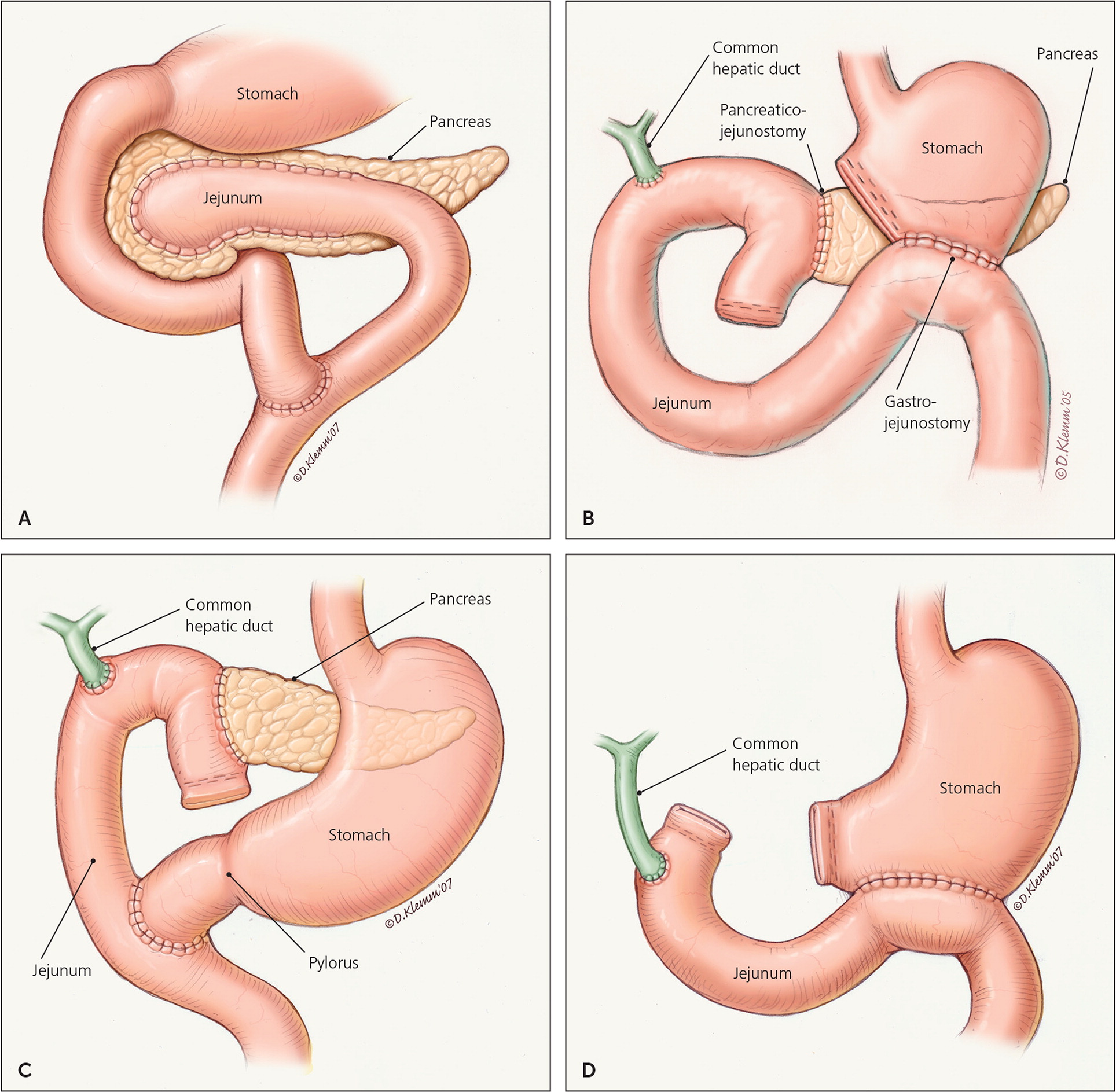
The most common decompression procedure is lateral pancreaticojejunostomy (Figure 3A15). This procedure is more effective at providing long-term pain relief (60% to 91% of patients) than endoscopic approaches.2,52–54 Surgery may also be performed to drain a symptomatic pseudocyst; however, because endoscopic approaches are as effective at relieving pain with fewer risks, surgery is reserved for when endoscopic intervention is ineffective or for complicated pseudocysts.47,48
Resection procedures 55,56 include pancreatoduodenectomy (Whipple procedure, Figure 3B15), pylorus-preserving pancreatoduodenectomy (Figure 3C15), duodenum-preserving pancreatic head resection (Beger or Frey procedures), and total pancreatectomy (Figure 3D15). The Whipple procedure is the most commonly performed surgery in patients with chronic pancreatitis because of its high success rate (pain relief in 85% of patients) and low mortality (less than 3%).8,55,56 Pancreatoduodenectomy is indicated with pancreatic head enlargement and typically results in significant pain relief.51 A Cochrane review of five trials including 292 participants compared the Whipple procedure with duodenum-preserving pancreatic head resection. Although hospital stay was shorter for duodenum-preserving pancreatic head resection, there was no statistical difference in mortality or quality of life.57,58 Total pancreatectomy is rarely performed because of high rates of complications and low rates of pain relief. Because of the high risk of diabetes in patients with chronic pancreatitis, total pancreatectomy should always be performed with an autologous islet cell transplant.56,59
Complications
Complications of chronic pancreatitis are summarized in Table 6.13,15,55,60 The most common causes of morbidity and mortality are chronic debilitating pain, diabetes, pancreatic pseudocysts, and pancreatic cancer. Although pain is often present at diagnosis, it takes roughly five years for diabetes to develop.12,13,55,61 Pancreatic pseudocysts are usually asymptomatic but may cause serious complications in 25% to 30% of patients with pancreatitis. Pseudocysts can lead to rupture, infection, bleeding, and obstruction.10,13,54,55 Recurrent attacks of acute pancreatitis can cause pancreatic abscesses and necrosis, sepsis, and multi-organ failure.18 Almost one-fourth of patients (23.4%) with chronic pancreatitis have osteoporosis and almost two-thirds (65%) have osteoporosis or osteopenia.60 There is a markedly increased risk of pancreatic cancer in patients with chronic pancreatitis, particularly among those with long-term alcohol use or hereditary pancreatitis.2,3,12,14,25–28
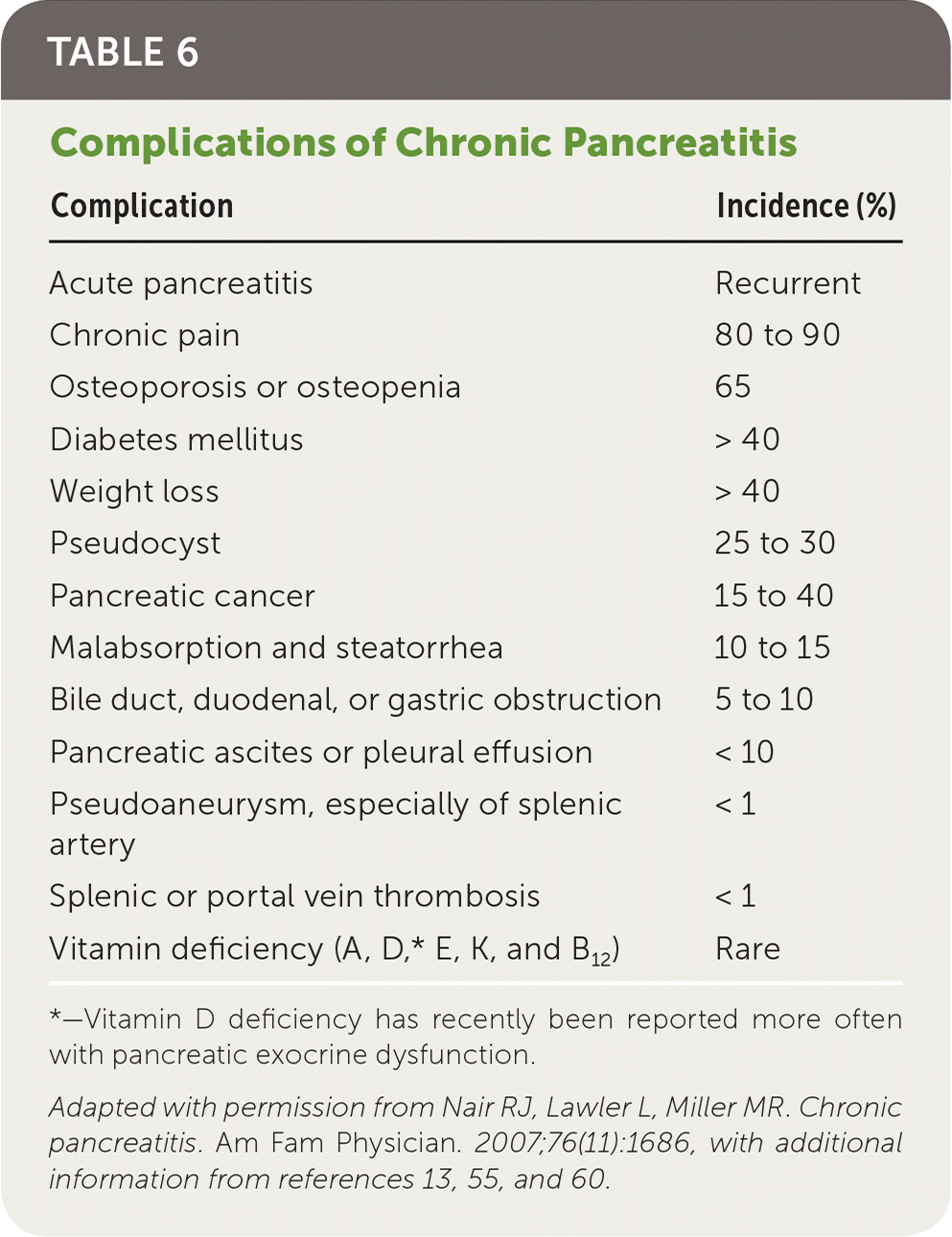
| Complication | Incidence (%) |
|---|---|
| Acute pancreatitis | Recurrent |
| Chronic pain | 80 to 90 |
| Osteoporosis or osteopenia | 65 |
| Diabetes mellitus | > 40 |
| Weight loss | > 40 |
| Pseudocyst | 25 to 30 |
| Pancreatic cancer | 15 to 40 |
| Malabsorption and steatorrhea | 10 to 15 |
| Bile duct, duodenal, or gastric obstruction | 5 to 10 |
| Pancreatic ascites or pleural effusion | < 10 |
| Pseudoaneurysm, especially of splenic artery | < 1 |
| Splenic or portal vein thrombosis | < 1 |
| Vitamin deficiency (A, D,* E, K, and B12) | Rare |
Surveillance
Patients with hereditary pancreatitis have a 10-fold increased risk of pancreatic cancer compared with the general population.61,62 Therefore, some experts suggest offering counseling and screening to patients with hereditary pancreatitis beginning at 40 years of age. The most recommended screening methods are EUS, CT, and endoscopic retrograde cholangiopancreatography; there is no consensus on screening interval. Additionally, patients with chronic pancreatitis who exhibit a change in clinical symptoms (pain, weight loss, jaundice) should be evaluated for neoplasm.63 All patients at increased risk of cancer should be counseled on mitigating risk factors, especially alcohol and tobacco use.62,63 If a malignancy is suspected, a triphasic, pancreas protocol CT is the standard for diagnosis. If this test is not available or if the patient has an allergy to contrast media, MRI with gadolinium may be used.62
This article updates a previous article on this topic by Nair, et al.15
Data Sources: A PubMed search was completed in Clinical Queries using the key terms chronic pancreatitis and pancreatitis. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. Also searched were the Agency for Healthcare Research and Quality evidence reports, Clinical Evidence, the Cochrane database, the Institute for Clinical Systems Improvement, the National Guideline Clearinghouse database, Dynamed, and Essential Evidence Plus. Search dates: March 18, 2016, and September 20, 2017.