A more recent article on cervical cancer screening is available.
This article has been updated to incorporate the August 2018 USPSTF recommendations on cervical cancer screening.
Am Fam Physician. 2018;97(7):441-448
Related article: Cervical Cancer: Evaluation and Management
Author disclosure: No relevant financial affiliations.
Screening in women has decreased the incidence and mortality of cervical cancer. Precancerous cervical lesions (cervical intraepithelial neoplasias) and cervical carcinomas are strongly associated with sexually-transmitted high-risk human papillomavirus (HPV) infection, which causes more than 99% of cervical cancers. Screening methods include cytology (Papanicolaou test) and HPV testing, alone or in combination. The American Academy of Family Physicians and the U.S. Preventive Services Task Force recommend starting screening in immunocompetent, asymptomatic women at 21 years of age. Women 21 to 29 years of age should be screened every three years with cytology alone. Women 30 to 65 years of age should be screened every five years with cytology plus HPV testing or every three years with cytology alone. Screening is not recommended for women younger than 21 years or in women older than 65 years with an adequate history of negative screening results. In 2015, the American Society for Colposcopy and Cervical Pathology and the Society of Gynecologic Oncology published interim guidance for the use of primary HPV testing, and the U.S. Preventive Services Task Force endorsed it in its 2018 guidelines. [updated]
Cervical cancer is responsible for more than 7% of all cancer-related deaths in women worldwide.1 Most cases of cervical cancer (85%) occur in developing countries that have ineffective screening programs.2 Total cancer-related deaths in American women declined by more than 80% from 1930 to 2012, primarily because of widespread use of cytology (Papanicolaou [Pap] test).3 The annual incidence and mortality rate of cervical cancer have decreased nearly 50% since 1975; there were reportedly 7.5 cases per 100,000 women from 2009 to 2013, and 2.3 deaths per 100,000 women in 2011.4,5 The most common types of cervical cancer are squamous cell carcinoma and adenocarcinoma.2 The American Cancer Society projected that there would be 12,820 new cases of cervical cancer diagnosed in 2017 in the United States, with 4,210 deaths.3
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Cervical cancer screening in women before 21 years of age leads to more harms than benefits and does not reduce cervical cancer incidence or mortality. | A | 4, 14, 16, 18, 20 |
| Average-risk women 21 to 29 years of age should be screened every three years with cytology alone every five years with primary HPV testing, or every five year with cotesting. [updated] | A | 4, 16, 18, 20 |
| Average-risk women 30 to 65 years of age should be screened every three years with cytology alone or every five years with a combination of cytology and HPV testing. | A | 4, 16, 18, 20 |
| Cervical cancer screening should be discontinued in women older than 65 years with an adequate history of negative screening results. | C | 4, 16, 18, 20 |
| Annual cervical cancer screening is not recommended for average-risk women of any age. | A | 4, 16, 18, 20 |
| Women with a hysterectomy unrelated to cancer should not be screened for cervical cancer. | C | 4, 16, 18, 20 |
| Women with a hysterectomy related to a history of cancer should be screened for cervical cancer for 20 years after the hysterectomy. | C | 4, 16, 18, 20 |
| Primary HPV testing may be considered for cervical cancer screening every three years in women 25 years and older. | B | 4, 15, 23 |
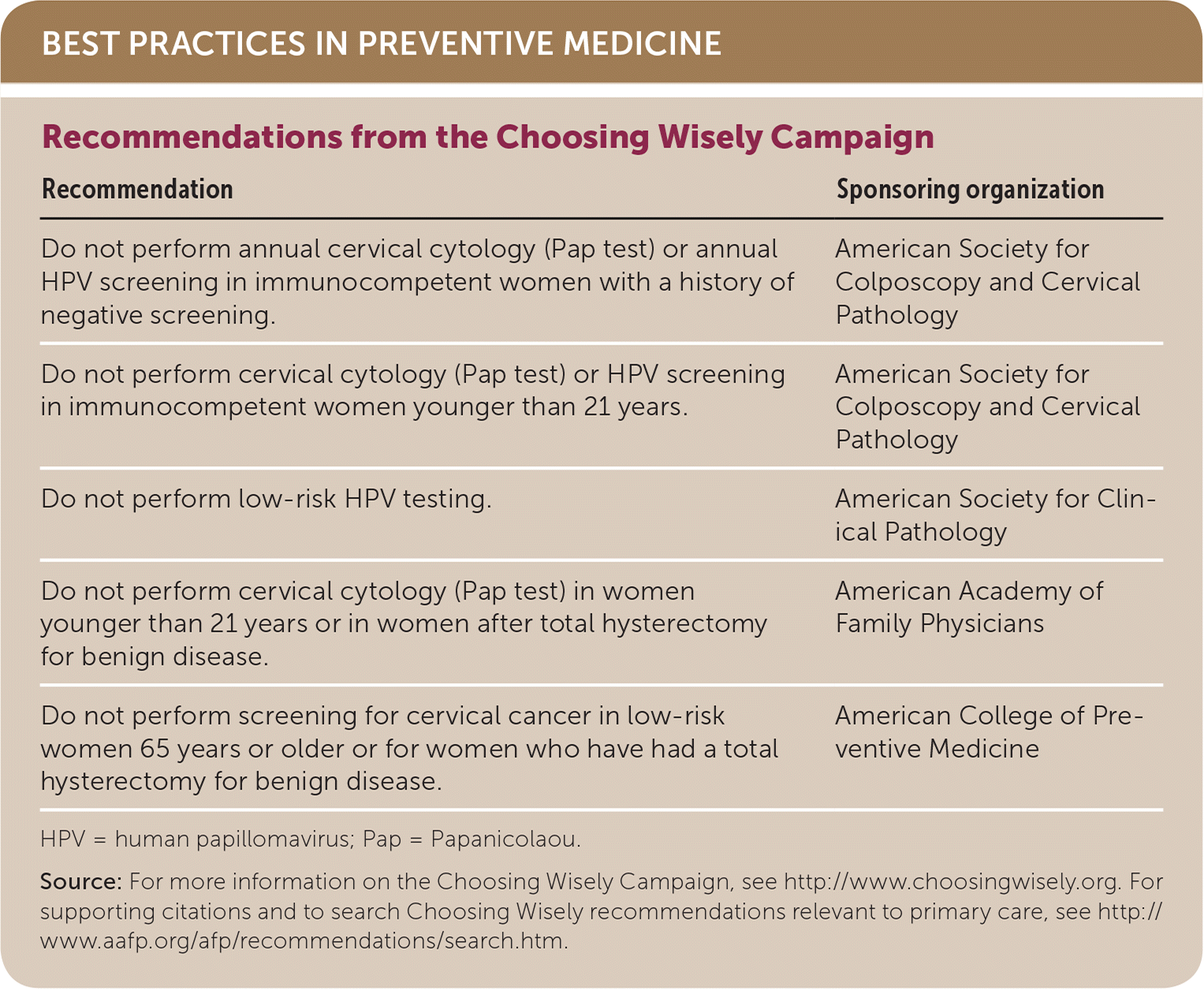
| Recommendation | Sponsoring organization |
|---|---|
| Do not perform annual cervical cytology (Pap test) or annual HPV screening in immunocompetent women with a history of negative screening. | American Society for Colposcopy and Cervical Pathology |
| Do not perform cervical cytology (Pap test) or HPV screening in immunocompetent women younger than 21 years. | American Society for Colposcopy and Cervical Pathology |
| Do not perform low-risk HPV testing. | American Society for Clinical Pathology |
| Do not perform cervical cytology (Pap test) in women younger than 21 years or in women after total hysterectomy for benign disease. | American Academy of Family Physicians |
| Do not perform screening for cervical cancer in low-risk women 65 years or older or for women who have had a total hysterectomy for benign disease. | American College of Preventive Medicine |
Nearly one-half of women with cervical cancer were not screened before diagnosis, and another 10% were not screened within the previous five years.4 Although the rates of cervical cancer in U.S. women who have adequate access to screening are decreasing, patients who lack regular preventive health care services continue to be at higher risk.6
Types of human papillomavirus (HPV) are categorized as low risk (wart-causing) and high risk (oncogenic, cancer-causing). Precancerous cervical lesions, called cervical intraepithelial neoplasias (CINs), and cervical carcinomas are strongly associated with sexually transmitted high-risk HPV infection, which causes more than 99% of cervical cancers.7 There are more than 200 types of HPV strains, of which about 40 types commonly infect the anogenital region.8 Types 16 and 18 are high-risk strains that cause 70% of all cervical cancers.8 Table 1 summarizes cervical cancer risk based on HPV genotype.4,8,9 Risk factors for high-risk HPV infection include early onset of sexual activity, multiple sex partners, long-term use of oral contraceptives, low socioeconomic status, micronutrient deficiency, immunosuppression, and tobacco use.2
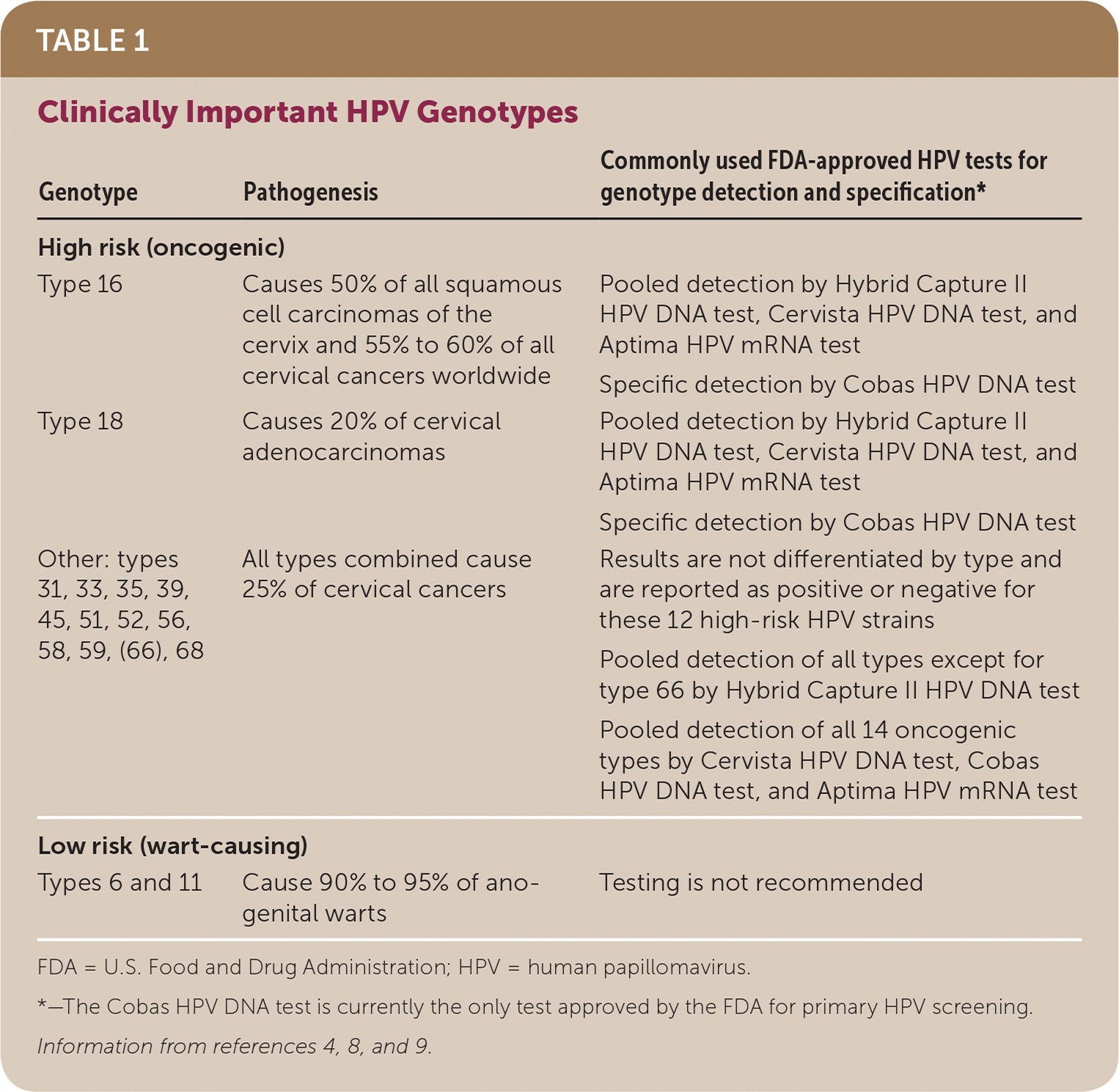
| Genotype | Pathogenesis | Commonly used FDA-approved HPV tests for genotype detection and specification* |
|---|---|---|
| High risk (oncogenic) | ||
| Type 16 | Causes 50% of all squamous cell carcinomas of the cervix and 55% to 60% of all cervical cancers worldwide | Pooled detection by Hybrid Capture II HPV DNA test, Cervista HPV DNA test, and Aptima HPV mRNA test |
| Specific detection by Cobas HPV DNA test | ||
| Type 18 | Causes 20% of cervical adenocarcinomas | Pooled detection by Hybrid Capture II HPV DNA test, Cervista HPV DNA test, and Aptima HPV mRNA test |
| Specific detection by Cobas HPV DNA test | ||
| Other: types 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, (66), 68 | All types combined cause 25% of cervical cancers | Results are not differentiated by type and are reported as positive or negative for these 12 high-risk HPV strains |
| Pooled detection of all types except for type 66 by Hybrid Capture II HPV DNA test | ||
| Pooled detection of all 14 oncogenic types by Cervista HPV DNA test, Cobas HPV DNA test, and Aptima HPV mRNA test | ||
| Low risk (wart-causing) | ||
| Types 6 and 11 | Cause 90% to 95% of anogenital warts | Testing is not recommended |
Most cervical HPV infections are transient, although a small percentage are persistent. It takes the immune system six to 24 months to clear a transient HPV infection.10 Factors that determine which HPV infections will persist are not entirely understood, but infection with HPV type 16 or 18 is more likely to persist and progress. High-risk HPV infection is more likely to resolve in younger women. Other HPV-induced cancers include vaginal, vulvar, anal, penile, and oropharyngeal cancers.4 It is unknown if previously resolved HPV infection produces immunity toward future infection with the same HPV genotype.
Screening with cytology can detect early cervical cancer precursors and early-stage disease. Precursors include atypical squamous cells of undetermined significance and low-grade squamous intraepithelial lesions on cytology, and mild dysplasia, also known as CIN1, on histology. Pre-cancerous cervical lesions include high-grade squamous intraepithelial lesions and atypical glandular cells on cytology, and CIN2 and CIN3 (i.e., moderate and severe dysplasia) on histology.
Cervical Cancer Screening Methods
Cervical cancer screening includes cytology and HPV testing, alone or in combination. Conventional cytology (a Pap test sample affixed to a slide at the time of testing) and liquid-based cytology (a newer method for collecting, transporting, and preparing cells collected by the Pap test in a liquid medium [e.g., ThinPrep Pap test]) provide comparable results. Both methods are acceptable and have nearly equivalent sensitivity and specificity for detection of high-grade CIN.4,11–14
HPV testing, alone or in combination with cytology, is more sensitive than cytology alone in detecting CIN2 and CIN3.15 There are a variety of tests approved by the U.S. Food and Drug Administration (FDA) for detecting cervical HPV, including HPV DNA and HPV mRNA tests (Table 14,8,9). Current methods for using cervical HPV testing in the United States include triage testing for patients with abnormal findings on cytology (reflex testing), adjunct testing with cytology (cotesting), and primary testing.
Screening Recommendations
The decision of when, how, and how often to screen for cervical cancer depends on a woman's age, screening history, risk factors, and the choice of screening tests available. Current screening recommendations from the U.S. Preventive Services Task Force, the American Academy of Family Physicians, and other national organizations are summarized in Table 24,15–21 and Table 3.4,16,18–20,22 Women with symptoms or visible cervical lesions on speculum examination should undergo diagnostic testing rather than screening.
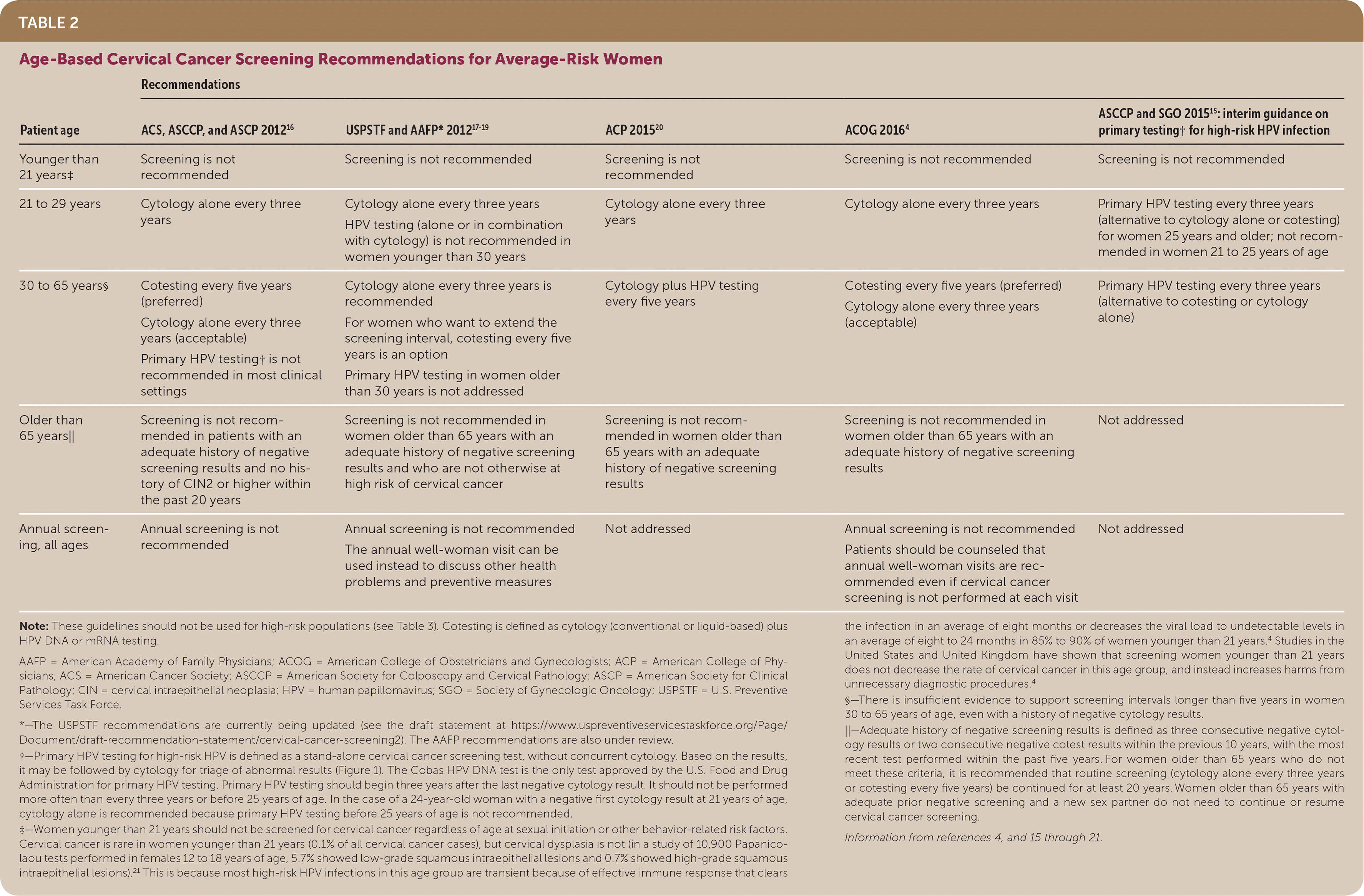
| Patient age | Recommendations | ||||
|---|---|---|---|---|---|
| ACS, ASCCP, and ASCP 201216 | USPSTF and AAFP* 201817–19 | ACP 201520 | ACOG 20164 | ASCCP and SGO 201515: interim guidance on primary testing† for high-risk HPV infection | |
| Younger than 21 years‡ | Screening is not recommended | Screening is not recommended | Screening is not recommended | Screening is not recommended | Screening is not recommended |
| 21 to 29 years | Cytology alone every three years | Cytology alone every three years HPV testing (alone or in combination with cytology) is not recommended in women younger than 30 years Primary HPV testing every five years (preferred) | Cytology alone every three years Primary HPV testing every three years with FDA-approved test per ASCCP/SGO interim guidance | Cytology alone every three years | Primary HPV testing every three years (alternative to cytology alone or cotesting) for women 25 years and older; not recommended in women 21 to 25 years of age |
| 30 to 65 years§ | Cotesting every five years (preferred) Cytology alone every three years (acceptable) Primary HPV testing† is not recommended in most clinical settings | Cytology alone every three years is recommended Cotesting every five years (acceptable) | Cytology plus HPV testing every five years | Cotesting every five years (preferred) Cytology alone every three years (acceptable) | Primary HPV testing every three years (alternative to cotesting or cytology alone) |
| Older than 65 years|| | Screening is not recommended in patients with an adequate history of negative screening results and no history of CIN2 or higher within the past 20 years | Screening is not recommended in women older than 65 years with an adequate history of negative screening results and who are not otherwise at high risk of cervical cancer; the annual "Pap visit" can be used instead to discuss other health problems and preventive measures | Screening is not recommended in women older than 65 years with an adequate history of negative screening results | Screening is not recommended in women older than 65 years with an adequate history of negative screening results | Not addressed |
| Annual screening, all ages | Annual screening is not recommended | Not addressed | Not addressed | Annual screening is not recommended Patients should be counseled that annual well-woman visits are recommended even if cervical cancer screening is not performed at each visit | Not addressed |
[updated]
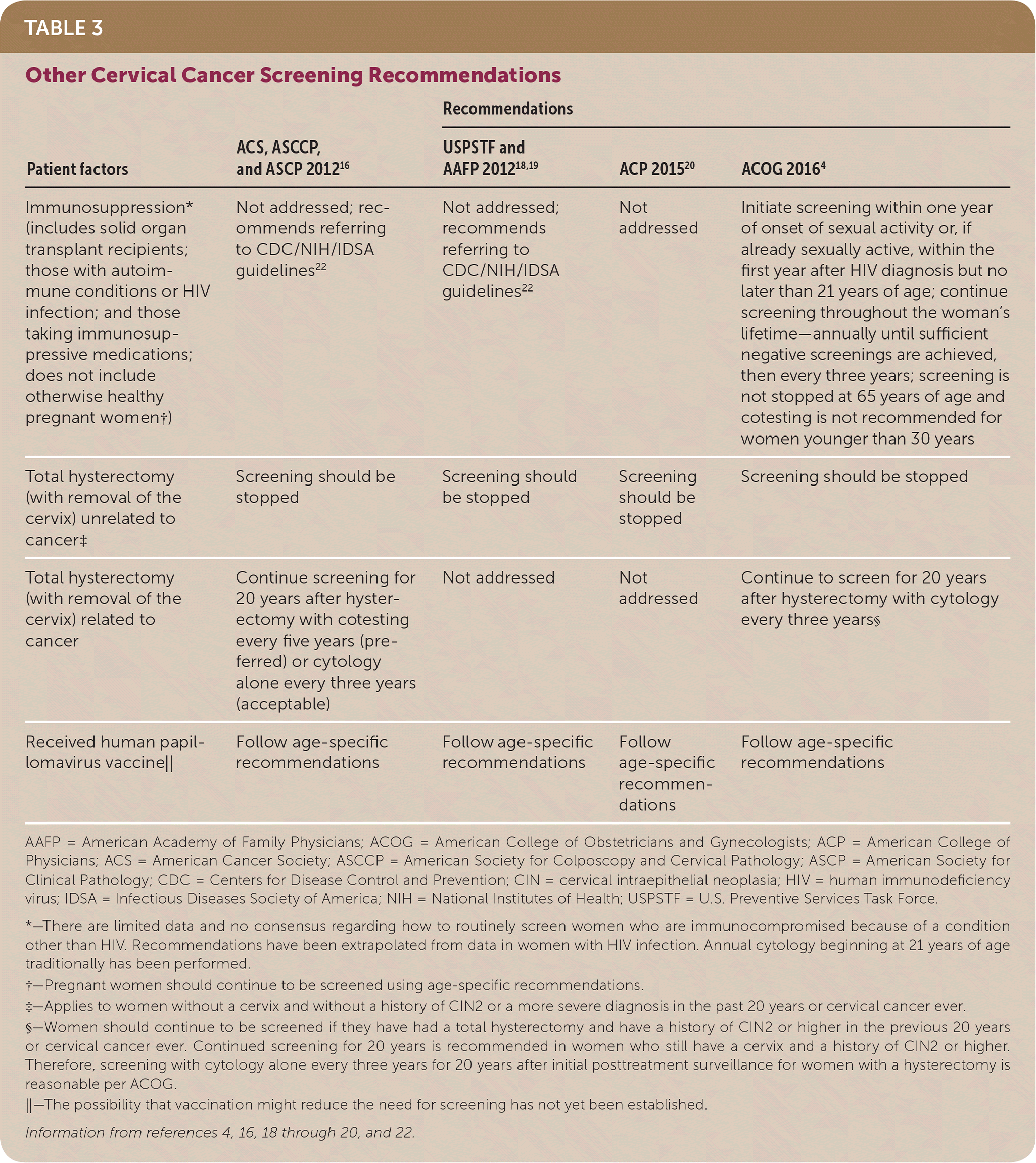
| Patient factors | ACS, ASCCP, and ASCP 201216 | Recommendations | ||
|---|---|---|---|---|
| USPSTF and AAFP 201818,19 | ACP 201520 | ACOG 20164 | ||
| Immunosuppression* (includes solid organ transplant recipients; those with autoimmune conditions or HIV infection; and those taking immunosuppressive medications; does not include otherwise healthy pregnant women†) | Not addressed; recommends referring to CDC/NIH/IDSA guidelines22 | Not addressed; recommends referring to CDC/NIH/IDSA guidelines22 | Not addressed | Initiate screening within one year of onset of sexual activity or, if already sexually active, within the first year after HIV diagnosis but no later than 21 years of age; continue screening throughout the woman's lifetime—annually until sufficient negative screenings are achieved, then every three years; screening is not stopped at 65 years of age and cotesting is not recommended for women younger than 30 years |
| Total hysterectomy (with removal of the cervix) unrelated to cancer‡ | Screening should be stopped | Screening should be stopped | Screening should be stopped | Screening should be stopped |
| Total hysterectomy (with removal of the cervix) related to cancer | Continue screening for 20 years after hysterectomy with cotesting every five years (preferred) or cytology alone every three years (acceptable) | Not addressed | Not addressed | Continue to screen for 20 years after hysterectomy with cytology every three years§ |
| Received human papillomavirus vaccine|| | Follow age-specific recommendations | Follow age-specific recommendations | Follow age-specific recommendations | Follow age-specific recommendations |
Primary HPV testing was not previously recommended, largely because of concerns about low specificity and insufficient data to determine when positive HPV test results require diagnostic evaluation.4 However, a large U.S. study, as well as four other large international randomized controlled trials (RCTs), have since shown that primary HPV screening has equivalent or superior effectiveness to cytology alone.4,15,18,23 [updated]
Additionally, an effective algorithm for managing patients with positive findings on primary HPV screening has been validated4,15,23 (Figure 115). As a result, the FDA approved the Cobas HPV DNA test in August 2014 for primary cervical cancer screening in women. In 2015, the American Society for Colposcopy and Cervical Pathology and the Society of Gynecologic Oncology provided interim guidance on the use of primary HPV testing which is also highlighted in the 2018 U.S. Preventive Services Task Force recommendation fro cervical cancer screening4,15,18 (Table 2).4,15–21 The U.S. Preventive Services Task Force is currently reviewing the evidence regarding primary HPV testing.18 The American Academy of Family Physicians suspended its Choosing Wisely recommendation against primary HPV testing in women younger than 30 years because of the uncertainty around the effectiveness of HPV testing alone.17 [updated]
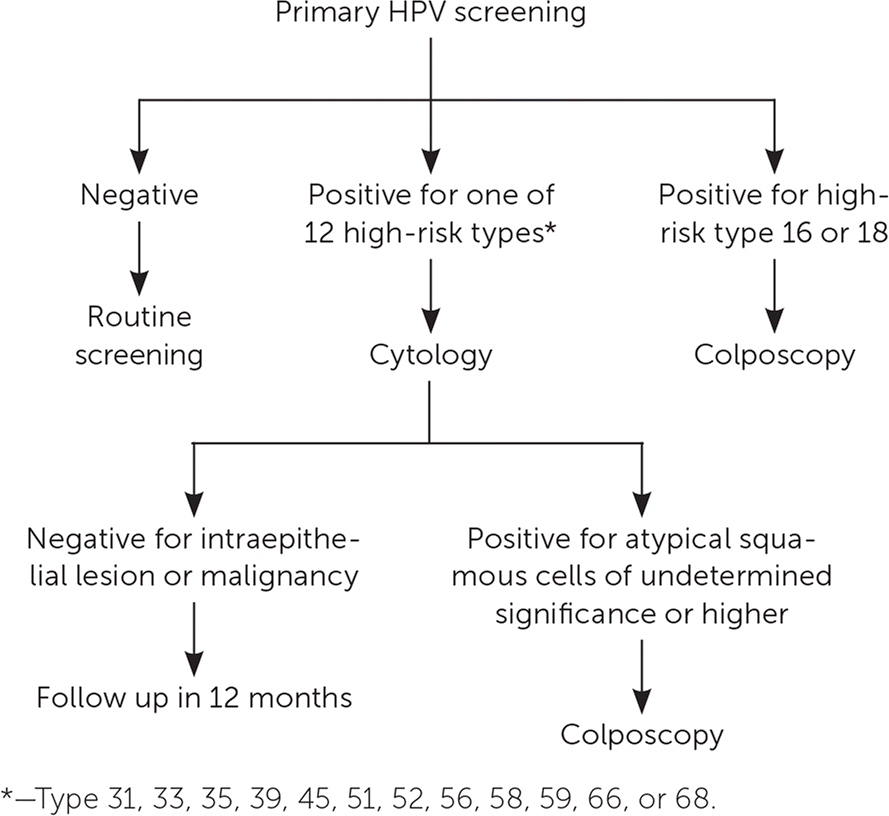
Primary HPV screening should not be performed in women younger than 25 years or older than 65 years, or in women who are immunocompromised.4 Rescreening after a negative primary HPV test result should occur no earlier than three years later, and patients with positive results should be tested for specific HPV genotype. If testing is negative for HPV types 16 and 18 but positive for other high-risk genotypes, then cytology should be performed. If cytology results are negative, then follow-up testing should be performed in 12 months (Figure 1).15 The type of follow-up testing to perform is not specified in the interim guideline, but the American College of Obstetricians and Gynecologists recommends cotesting.4,15
RCTs show that primary HPV testing in women younger than 30 years leads to a higher incidence of transient HPV infection, resulting in increased CIN3 detection and subsequent treatment intervention.18 [updated] The actual impact on cervical cancer prevention in this age group needs further investigation.15 Some believe that the harms of excessive diagnostic testing will outweigh the benefits.23 Primary HPV testing is a rapidly evolving area of preventive medicine. There are ongoing international RCTs investigating primary HPV testing, as well as studies of patient-collected HPV samples.23–29 [updated] Primary polymerase chain reaction–based HPV testing via self-sampling has similar sensitivity to that of an in-office collection method, with reportedly wider acceptability and positive feedback, especially in underscreened and unscreened patient populations.24–29
Weighing the Benefits and Harms of Screening
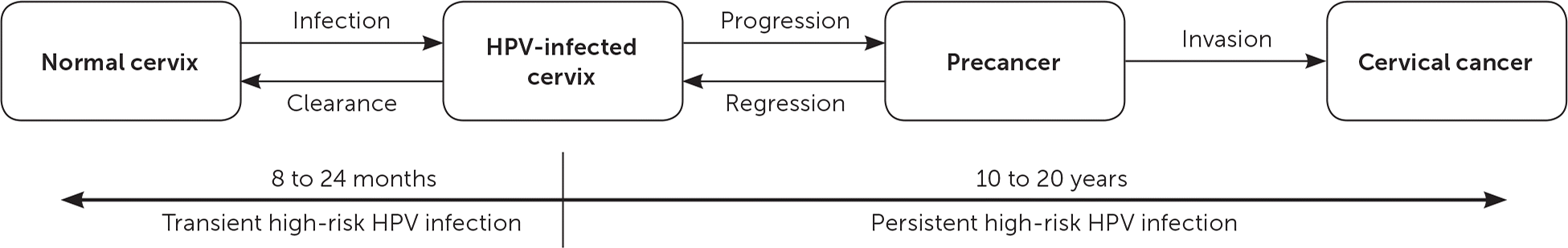
The primary goal of cervical cancer screening is to decrease mortality by detecting precancerous lesions and intervening to prevent the progression to cervical cancer. It is important to recognize that the risk of HPV infection is highest among those who are newly sexually active, and that the risk decreases with age.20 The peak incidence of high-risk HPV infection is in teenagers and in women in their early 20s.4 The progression from persistent high-risk HPV infection to invasive cervical disease takes an average of 10 to 20 years (Figure 2).30 Because of this slow oncogenesis, persistent high-risk HPV infections that manifest as abnormalities of the cervix can be detected early, resulting in less-invasive treatments and overall fewer adverse outcomes.18
The optimal cervical cancer screening program maximizes the benefit to women and minimizes harms and the costs of screening. Harms are related to both screening tests and the procedures required for diagnosis, management, and follow-up of patients with abnormal screening results.20 Screening tests and subsequent diagnosis and management can lead to psychological harms such as distress and anxiety.18,20 The pelvic examination and Pap test can cause mild bleeding and cramping in some women. Additionally, inadequate sampling may necessitate repeat procedures. Abnormal screening test results can lead to more frequent testing, out-of-pocket expenses, and invasive procedures such as colposcopy with cervical biopsy.18
The harms of diagnostic testing with colposcopy or endocervical curettage include bleeding, pain, infection, and failure to diagnose from inadequate sampling.18 Short-term risks of treatment methods include bleeding, pain, and infection. Long-term risks of treatment include subsequent preterm delivery and neonatal mortality because of severe prematurity.20 Overdiagnosis and overtreatment are common; the treatment threshold in the United States is CIN2, and about 40% of CIN2 lesions regress over six months without intervention.20
To reduce harms from cervical cancer screening, guidelines recommend against screening women before 21 years of age and in patients who have had a hysterectomy for reasons unrelated to cancer.4,16,18,20 Screening should be stopped after 65 years of age in women with an adequate history of negative screening results.16,20 Annual screening is not recommended for average-risk women of any age; screening too frequently has been proven to have greater harms than benefits.4,16,18,20
Prevention
Preventing high-risk HPV infection is the key to the prevention of cervical dysplasia and cancer. Barrier contraceptives, such as condoms, are only about 70% effective at preventing HPV transmission.9 In 2016, the Centers for Disease Control and Prevention changed the recommendations for HPV vaccination to include vaccinating boys and girls before 15 years of age, and as early as nine years of age.31 A two-dose series is used when initiated before 15 years of age, whereas a three-dose series is required if initiated at 15 years or older or if the individual is immunocompromised. Vaccination is recommended through 26 years of age for females and 21 years of age for males.31
Gardasil-9 (9-valent) is the only FDA-approved HPV vaccine (eTable A). If Cervarix (bivalent, no longer available in the United States) or Gardasil (quadrivalent, no longer available in the United States) has already been given, there is no evidence that the patient should be revaccinated using Gardasil-9. Additionally, the HPV vaccination series should be finished with whichever vaccine type is available.4 Because long-term effectiveness of the vaccine is unknown, all patients with a cervix should undergo age-based cervical cancer screening regardless of vaccination status.4
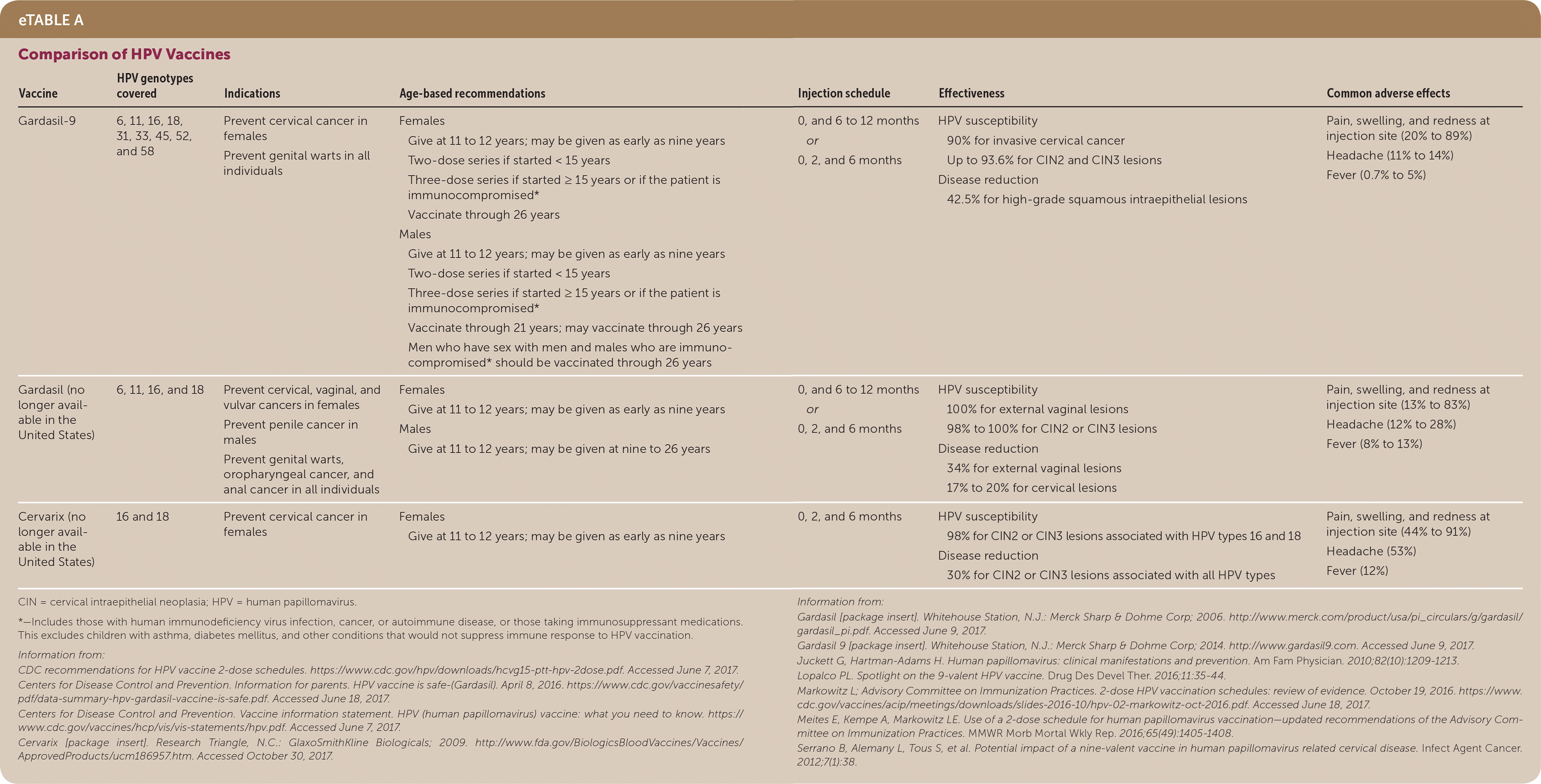
| Vaccine | HPV genotypes covered | Indications | Age-based recommendations | Injection schedule | Effectiveness | Common adverse effects |
|---|---|---|---|---|---|---|
| Gardasil-9 | 6, 11, 16, 18, 31, 33, 45, 52, and 58 | Prevent cervical cancer in females Prevent genital warts in all individuals | Females
| 0, and 6 to 12 months or 0, 2, and 6 months |
| Pain, swelling, and redness at injection site (20% to 89%) Headache (11% to 14%) Fever (0.7% to 5%) |
| Gardasil (no longer available in the United States) | 6, 11, 16, and 18 | Prevent cervical, vaginal, and vulvar cancers in females Prevent penile cancer in males Prevent genital warts, oropharyngeal cancer, and anal cancer in all individuals |
| 0, and 6 to 12 months or 0, 2, and 6 months |
| Pain, swelling, and redness at injection site (13% to 83%) Headache (12% to 28%) Fever (8% to 13%) |
| Cervarix (no longer available in the United States) | 16 and 18 | Prevent cervical cancer in females |
| 0, 2, and 6 months |
| Pain, swelling, and redness at injection site (44% to 91%) Headache (53%) Fever (12%) |
Data Sources: A PubMed search was conducted using the key terms cervical intraepithelial neoplasia, low-grade squamous intraepithelial lesion, Pap smear, cytology, colposcopy, human papillomavirus, HPV, HPV testing, and HPV vaccination. The search included retrospective studies, prospective studies, meta-analyses, and reviews. Essential Evidence Plus, Google Scholar, the National Institute for Health and Care Excellence guidelines, and the Cochrane database were also searched. Search dates: August 2016, February 2017, May 2017, and October 2017.
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of Defense, the U.S. Army Medical Department, or the U.S. Army Service at large.