Am Fam Physician. 2018;97(7):449-454
Related article: Cervical Cancer Screening
Author disclosure: No relevant financial affiliations.
Human papillomavirus infection is the precursor for the development of cervical cancer and is detectable in 99.7% of squamous cell carcinoma and adenocarcinoma cases. Early detection of precancerous lesions with Papanicolaou testing remains the primary mechanism for cancer prevention. Once cervical cancer is diagnosed, treatment may involve surgery, radiation therapy, chemotherapy, or a combination. The choice of therapy depends on the stage of disease, lymph node involvement, patient comorbidities, and risk factors for recurrence. Early-stage, microinvasive disease may be treated with surgery alone if margins are negative and there is no lymph node involvement; adjuvant chemoradiation should be considered for other early-stage disease. Locally advanced disease is often treated with chemoradiation. The addition of bevacizumab, an antivascular endothelial growth factor monoclonal antibody, to combination chemotherapy improves survival among patients with recurrent, persistent, or metastatic cervical cancer. Disease stage and lymph node involvement are the most prognostic factors. Pregnancy status and desire to preserve fertility should be considered when developing a treatment strategy. After treatment, close follow-up with a gynecologist-oncologist for pelvic examinations at regular intervals is recommended to assess for recurrence.
Every year, nearly 13,000 cases of cervical cancer are diagnosed, with more than 4,000 deaths.1 Although the highest incidence of cervical cancer is among women 40 to 49 years of age (14 cases per 100,000 women per year), 40% of women are older than 40 years at diagnosis.2 Rates of cervical cancer are higher in the southern region of the United States.2 There are significant disparities in cervical cancer–related mortality among racial and ethnic groups. Black women are more than twice as likely to die from cervical cancer than white women.3 Mortality rates are also higher among Hispanic women.1
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Cervical cancer is staged clinically before surgery, based on tumor size, depth of invasion, spread into surrounding tissue, and distant metastases. | C | 21 |
| Adjuvant platinum-based chemoradiation should be considered after surgery for women with early cervical cancer (stage IA2–IIA) and risk factors for recurrence because it has been shown to reduce mortality. | A | 26 |
| Adding bevacizumab (Avastin) to combination chemotherapy should be considered for women with recurrent, persistent, or metastatic cervical cancer because it has been shown to improve overall survival. | B | 32 |
| Patients with cervical cancer should be referred to regional cancer centers with centralization of services. | B | 33 |

| Recommendation | Sponsoring organization |
|---|---|
| Avoid routine imaging for cancer surveillance in women with gynecologic cancer, specifically ovarian, endometrial, cervical, vulvar, and vaginal cancers. | Society of Gynecologic Oncology |
Pathogenesis
HPV is detected in 99.7% of squamous cell carcinomas and adenocarcinomas,6 the most common types of cervical cancer (Table 1).4 There are 15 known oncogenic strains of HPV, with types 16 and 18 involved in 70% of cervical cancer cases.7 Most sexually active adults will be exposed to HPV, and more than 50% of adults 20 to 24 years of age are currently infected.8 However, the immune system clears the virus within six months in 50% of women with the infection and within two years in up to 90% of women.9,10
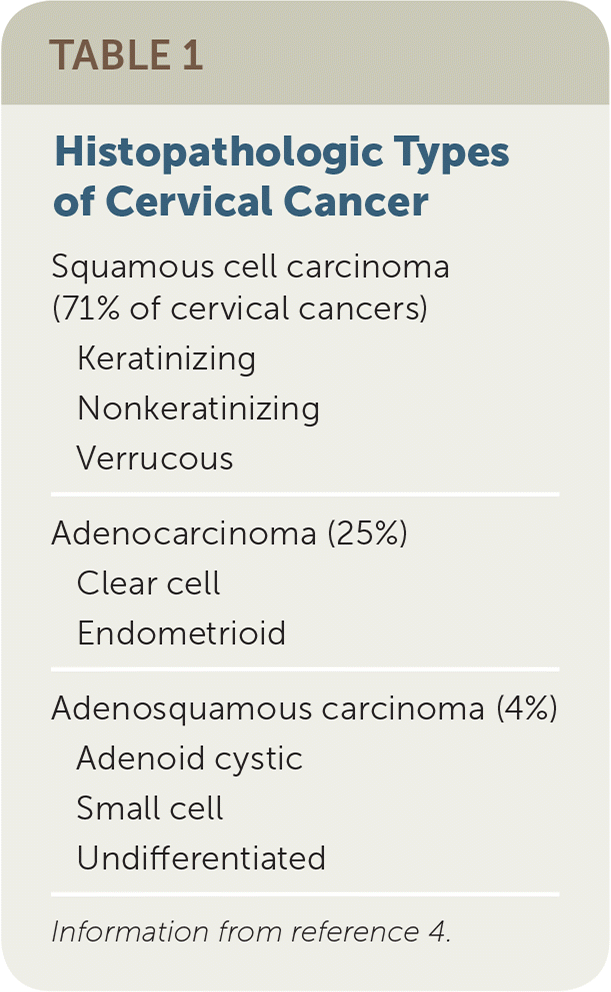
| Squamous cell carcinoma (71% of cervical cancers) | |
| Keratinizing | |
| Nonkeratinizing | |
| Verrucous | |
| Adenocarcinoma (25%) | |
| Clear cell | |
| Endometrioid | |
| Adenosquamous carcinoma (4%) | |
| Adenoid cystic | |
| Small cell | |
| Undifferentiated | |
When HPV infection persists in the metaplastic epithelium of the cervical transformation zone, it can lead to dysplastic cellular changes. Although low-grade dysplasia (cervical intraepithelial neoplasia 1 [CIN1]) usually regresses, it can progress to high-grade dysplasia (CIN2 or CIN3).11 Cervical cancer occurs when high-grade lesions extend beyond the basement membrane of the cervical epithelium. Approximately 20% of women with high-grade dysplasia will develop invasive cervical cancer within five years if left untreated.12
Risk Factors
Most cervical cancer risk factors relate to increased exposure to HPV or decreased ability to clear the virus immunologically.13 Smoking is associated with an increased risk of squamous cell carcinoma but not adenocarcinoma. This risk decreases by one-half 10 years after smoking cessation.14 Evidence suggests a genetic susceptibility to cervical cancer, and several genetic alterations have been implicated.15
In addition, a large prospective cohort study found that use of oral contraceptives for five to nine years was associated with increased risk of cervical cancer (hazard ratio = 2.0; 95% confidence interval, 1.3 to 3.0). The mechanism appears to be hormonal rather than behavioral and may be due to the effect of sex steroids on oncogene expression.16 The risk appears to decline after cessation of oral contraceptives. However, CIN is not currently a contraindication to oral contraceptive use per the U.S. Medical Eligibility Criteria or cervical cancer treatment guidelines.17 Table 2 includes risk factors for cervical squamous cell carcinoma.1,13–16,18,19
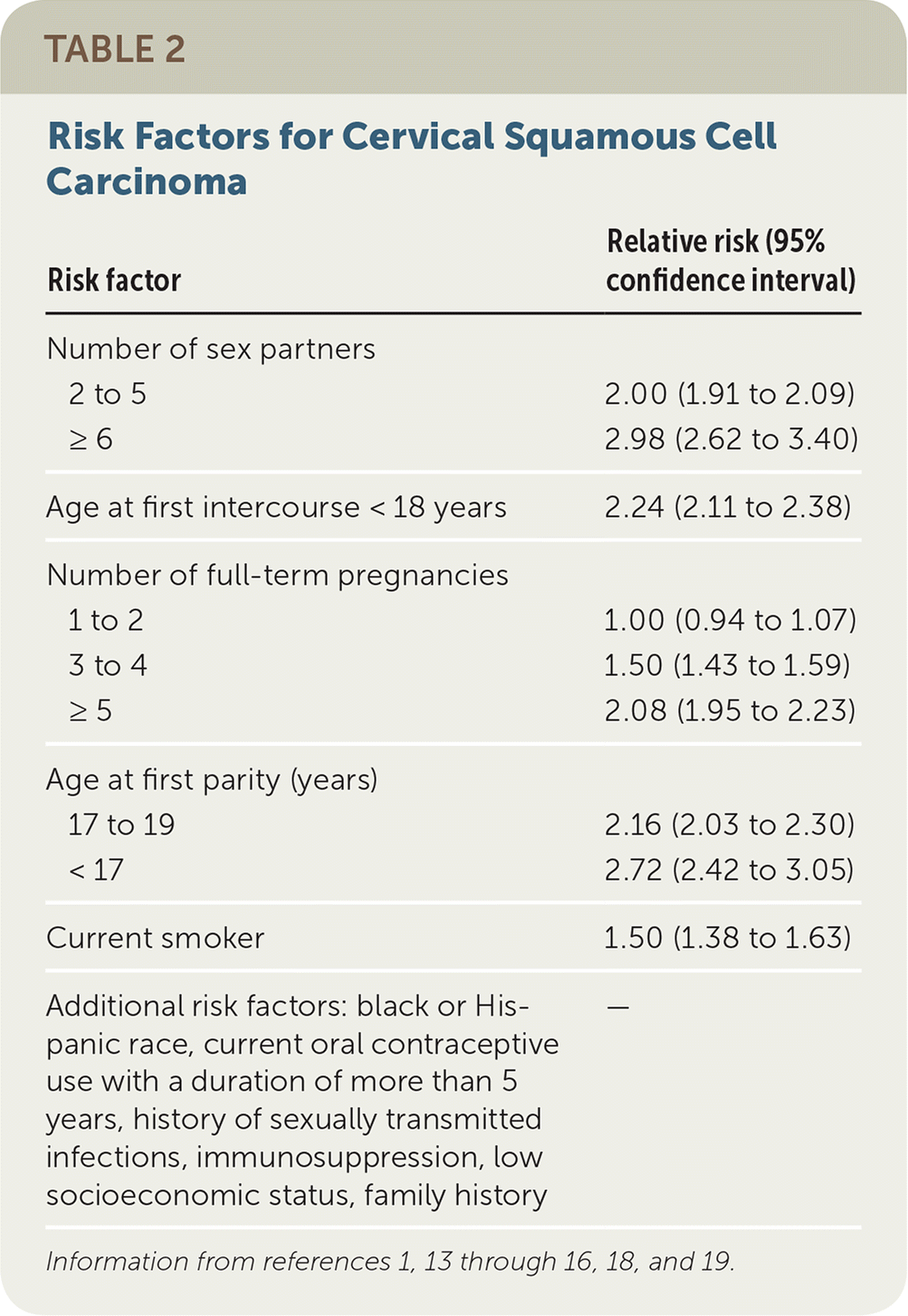
| Risk factor | Relative risk (95% confidence interval) | |
|---|---|---|
| Number of sex partners | ||
| 2 to 5 | 2.00 (1.91 to 2.09) | |
| ≥ 6 | 2.98 (2.62 to 3.40) | |
| Age at first intercourse < 18 years | 2.24 (2.11 to 2.38) | |
| Number of full-term pregnancies | ||
| 1 to 2 | 1.00 (0.94 to 1.07) | |
| 3 to 4 | 1.50 (1.43 to 1.59) | |
| ≥ 5 | 2.08 (1.95 to 2.23) | |
| Age at first parity (years) | ||
| 17 to 19 | 2.16 (2.03 to 2.30) | |
| < 17 | 2.72 (2.42 to 3.05) | |
| Current smoker | 1.50 (1.38 to 1.63) | |
| Additional risk factors: black or Hispanic race, current oral contraceptive use with a duration of more than 5 years, history of sexually transmitted infections, immunosuppression, low socioeconomic status, family history | — | |
Diagnosis
Cervical cancer may be detected after a Pap test result is abnormal, a lesion is visualized on pelvic examination, or clinical symptoms develop. Management of abnormal Pap test results should follow the American Society for Colposcopy and Cervical Pathology guideline.20 If colposcopy is indicated because of an abnormal Pap test result or visible lesion, cervical cancer may be diagnosed by colposcopy-directed biopsy or by an excisional procedure.
Although early cervical cancer is usually asymptomatic, it may cause abnormal uterine bleeding or postcoital bleeding. Advanced lesions can cause bladder outlet obstruction, resulting in bladder or bowel symptoms, back or pelvic pain, hematuria, or renal failure. On pelvic examination, cervical cancer may manifest as superficial ulceration, an exophytic tumor, or an enlarged, indurated cer vix. Any abnormal lesion visible on the cervix should be biopsied regardless of cervical cytology. Adenocarcinomas may not lead to an identifiable lesion if located in the endocervical canal.
Staging
Cervical cancer spreads via direct extension, and lymphatic and hematogenous routes. Staging is determined using the International Federation of Gynecology and Obstetrics 2014 guideline (Table 3).21,22 Cervical cancer is the only gynecologic cancer staged clinically before surgery, based on tumor size, depth of invasion, spread into surrounding tissue, and distant metastases (usually lung, liver, and bone).21 In suspected microinvasive disease, conization is used to determine depth of invasion.
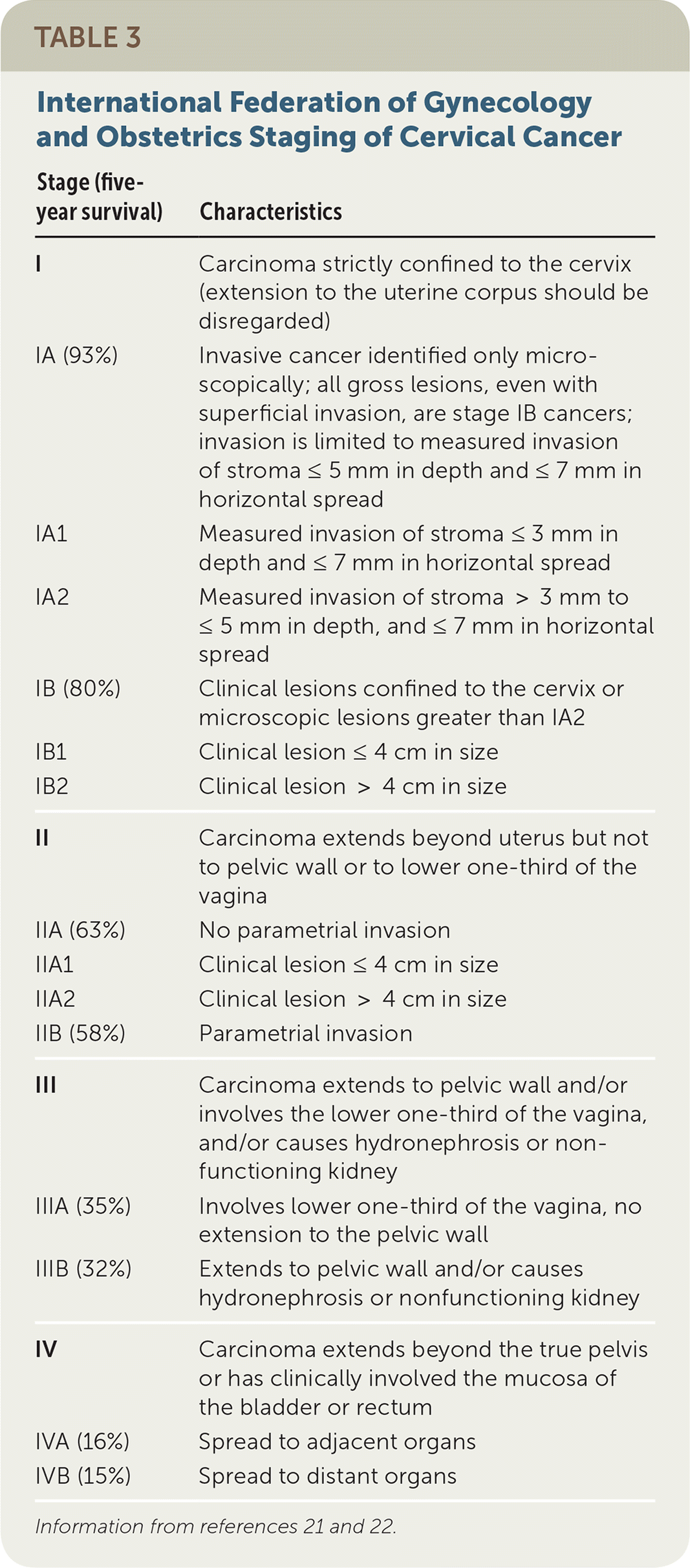
| Stage (five-year survival) | Characteristics |
|---|---|
| I | Carcinoma strictly confined to the cervix (extension to the uterine corpus should be disregarded) |
| IA (93%) | Invasive cancer identified only microscopically; all gross lesions, even with superficial invasion, are stage IB cancers; invasion is limited to measured invasion of stroma ≤ 5 mm in depth and ≤ 7 mm in horizontal spread |
| IA1 | Measured invasion of stroma ≤ 3 mm in depth and ≤ 7 mm in horizontal spread |
| IA2 | Measured invasion of stroma > 3 mm to ≤ 5 mm in depth, and ≤ 7 mm in horizontal spread |
| IB (80%) | Clinical lesions confined to the cervix or microscopic lesions greater than IA2 |
| IB1 | Clinical lesion ≤ 4 cm in size |
| IB2 | Clinical lesion > 4 cm in size |
| II | Carcinoma extends beyond uterus but not to pelvic wall or to lower one-third of the vagina |
| IIA (63%) | No parametrial invasion |
| IIA1 | Clinical lesion ≤ 4 cm in size |
| IIA2 | Clinical lesion > 4 cm in size |
| IIB (58%) | Parametrial invasion |
| III | Carcinoma extends to pelvic wall and/or involves the lower one-third of the vagina, and/or causes hydronephrosis or non-functioning kidney |
| IIIA (35%) | Involves lower one-third of the vagina, no extension to the pelvic wall |
| IIIB (32%) | Extends to pelvic wall and/or causes hydronephrosis or nonfunctioning kidney |
| IV | Carcinoma extends beyond the true pelvis or has clinically involved the mucosa of the bladder or rectum |
| IVA (16%) | Spread to adjacent organs |
| IVB (15%) | Spread to distant organs |
Additional diagnostic studies may aid in determining spread and prognosis, and in planning treatment. Computed tomography or magnetic resonance imaging assesses tumor size and spread, and computed tomography with or without positron emission tomography is used to evaluate lymphovascular space invasion (LVSI).
Management
Management of cervical cancer depends on stage, lymph node involvement, patient comorbidities, and risk factors for recurrence.23 Treatment may include surgical resection, radiation, chemotherapy, or a combination. There are several types of hysterectomy procedures that are used to treat cervical cancer depending on stage (Table 4), and a minimally invasive approach (laparoscopic or robotic) is often possible.24 Microinvasive disease (stage IA1) and no LVSI may be treated with simple hysterectomy if margins are negative.25 Women with early disease (stage IA–IB1) may be treated with radical hysterectomy and pelvic lymphadenectomy. A 2016 Cochrane review of 401 women with early disease (stage IA2–IIA) and risk factors for recurrence found that after surgery, adjuvant platinum-based chemoradiation significantly reduced mortality compared with radiation alone (hazard ratio = 0.56; 95% confidence interval, 0.36 to 0.87).26 Women who are not surgical candidates may be treated with primary radiation therapy or chemoradiation.
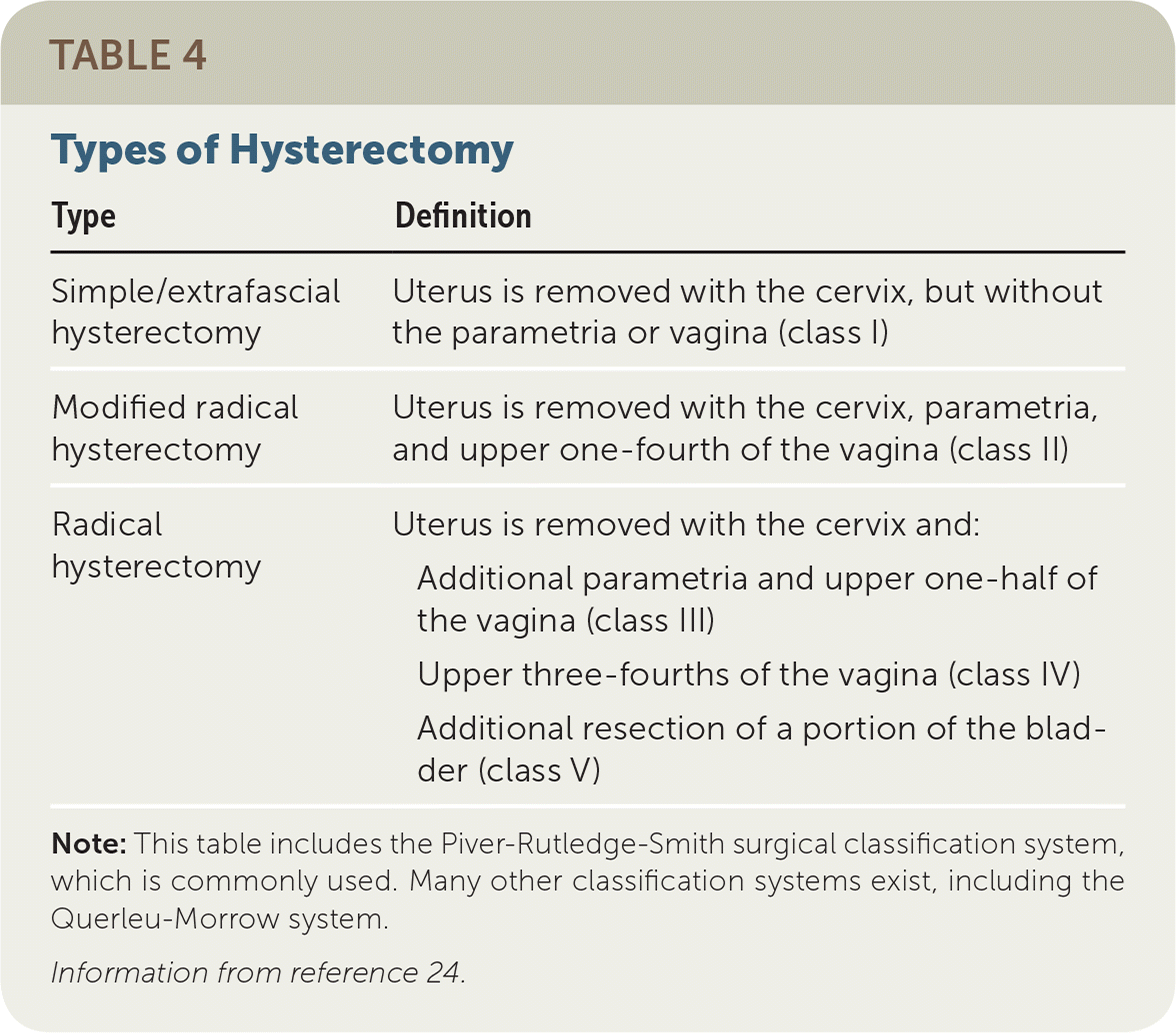
| Type | Definition |
|---|---|
| Simple/extrafascial hysterectomy | Uterus is removed with the cervix, but without the parametria or vagina (class I) |
| Modified radical hysterectomy | Uterus is removed with the cervix, parametria, and upper one-fourth of the vagina (class II) |
| Radical hysterectomy | Uterus is removed with the cervix and:
|
Locally advanced cervical cancer is often treated with primary chemoradiation.27 Currently, treatment is based on combination therapy with platinum-based regimens. Radiation involves external beam and high-dose intracavity brachytherapy.28 Posttreatment hysterectomy is not associated with increased survival rates and therefore is generally not recommended.29 However, hysterectomy may be performed in patients who have large, bulky tumors or high posttreatment tumor volumes.
Treatment of recurrent disease includes medical or surgical options. For women with local recurrence, surgical resection with hysterectomy or pelvic exenteration is an option. Exenteration is an ultraradical surgical procedure that involves en bloc removal of female reproductive organs, the lower urinary tract, and a portion of the rectosigmoid. Exenteration is generally used for women who have had previous unsuccessful radiation treatment, with or without hysterectomy, and it has a 50% cure rate.30 However, the procedure has a 3% to 5% mortality rate, 50% of patients have major complications, and there are no controlled trials to evaluate its effectiveness.31
Women with advanced metastatic disease are treated with chemotherapy (and radiation if not previously offered). Bevacizumab (Avastin) is an antivascular endothelial growth factor monoclonal antibody that inhibits tumor angiogenesis. A 2017 double-blind, randomized trial of 452 patients with recurrent, persistent, or metastatic cervical cancer found that adding bevacizumab to combination chemotherapy was associated with a 23% improvement in overall survival (hazard ratio = 0.77; 95% confidence interval, 0.62 to 0.95; P = .007) and an extension of median overall survival from 13.3 months to 16.8 months compared with combination chemotherapy alone. This trial confirms persisting benefit of bevacizumab that was previously shown in an interim survival analysis.32
Physicians should consider referring patients to regional cancer centers. A 2012 Cochrane review found that women with gynecologic cancer had prolonged survival when treated at facilities with centralization of services, such as regional cancer centers or academic institutions, compared with women treated at community hospitals.33
Prognosis
Prognosis is impacted by stage, tumor volume, depth of cervical stromal invasion, metastases, and LVSI (Table 321,22). Disease stage and lymph node status are the two most prognostic factors. For example, women with stage IA disease have a 93% five-year survival rate,22 but LVSI reduces this by approximately 50%.34
Adenocarcinoma
The incidence of adenocarcinoma has increased by 35% over the past 35 years.4 Although risk factors, diagnosis, staging, and treatment of adenocarcinoma are generally the same as for squamous cell carcinoma, there are some additional considerations. Ovarian metastases are more common with adenocarcinoma, and thus oophorectomy is recommended in early-stage disease.35 Larger adenocarcinomas (greater than 2 cm) are more likely to involve lymph nodes and have higher recurrence rates compared with squamous cell carcinomas of the same size.36
Fertility Preservation
Fertility-sparing options are available for women with early-stage cervical cancer. Stage IA1 can be treated with conization if margins are negative. More advanced disease can be treated with radical trachelectomy (removal of the cervix) and pelvic lymph node dissection. Fertility-sparing treatments for disease beyond stage IB1 and greater than 2 cm are not recommended.25 Women should be counseled regarding future pregnancy risk, including preterm birth, and need for obstetric consultation.
Pregnancy
Colposcopy without endocervical curettage is safe during pregnancy and preferred at 20 weeks' gestation if indicated based on the American Society for Colposcopy and Cervical Pathology guideline for managing an abnormal Pap test result.37 Stage IA1 disease may be treated with conization.25 In women with more advanced disease, management should be individualized based on stage, gestational age, and desire for continuation of the pregnancy. Imaging for staging should consider risk of fetal exposure to radiation and contrast media, and the value of diagnostic accuracy of the test compared with potentially safer studies.
Recurrence
Recurrence of cervical cancer occurs locoregionally or as metastatic disease, usually within three years of treatment.38 Therefore, initial close follow-up with a gynecologist-oncologist for pelvic examinations at regular three- to six-month intervals is recommended for the first two to five years following treatment, depending on risk of recurrence. Women with local recurrence may present with vaginal discharge, vaginal bleeding, dyspareunia, or pelvic pain. On physical examination, physicians may palpate or visualize a mass at the vaginal cuff. Metastatic symptoms may be vague or related to the affected site, such as bone pain. Imaging is not recommended unless otherwise indicated based on symptoms or physical examination findings.
After regular follow-up with a gynecologist-oncologist, low-risk women with early-stage disease may resume care with their primary care physicians after two to three years. Annual cytology is performed for detection of lower genital tract dysplasia, which occurs more often in women with a history of cervical cancer.39 Cervical cancer survivors should receive annual pelvic examinations to monitor for local recurrence and radiation-induced pelvic cancer.38,40
Patient Counseling
Patients should be educated about symptoms of potential recurrence and adverse effects of cervical cancer treatments (Table 5). Patients undergoing treatment may struggle with sexual, bowel, and urinary dysfunction, especially after radiation.27 Women with ovarian failure may be treated with hormone therapy; however, there is limited evidence of its safety in cervical cancer survivors.
| Symptoms of local recurrence: vaginal discharge, vaginal bleeding, dyspareunia, pelvic pain |
| Sexual health: vaginal lubrication, vaginal dilators after radiation, pelvic floor therapy, psychotherapy |
| Long-term effects of treatment: cystitis, proctitis, ovarian failure, chronic pelvic pain |
| Smoking cessation |
| Depression screening |
| Support resources: American Cancer Society (https://www.cancer.org/treatment/support-programs-and-services.html), Cancer Survivors Network (https://csn.cancer.org) |
This article updates a previous article on this topic by Canavan and Doshi.41
Data Sources: General searches were done using Essential Evidence Plus, UpToDate, DynaMed, the U.S. Preventive Services Task Force website, and the Cochrane database. The American Society for Colposcopy and Cervical Pathology, Journal of Lower Genital Tract Disease, and Obstetrics and Gynecology websites were also searched for disease-specific information and guidelines. A PubMed search was completed in Clinical Queries using the key terms cervical cancer, fertility, pregnancy, and human papillomavirus. Search dates: January 5 through November 10, 2017.