This is a corrected version of the article that appeared in print.
Am Fam Physician. 2018;97(7):463-464
Author disclosure: No relevant financial affiliations.

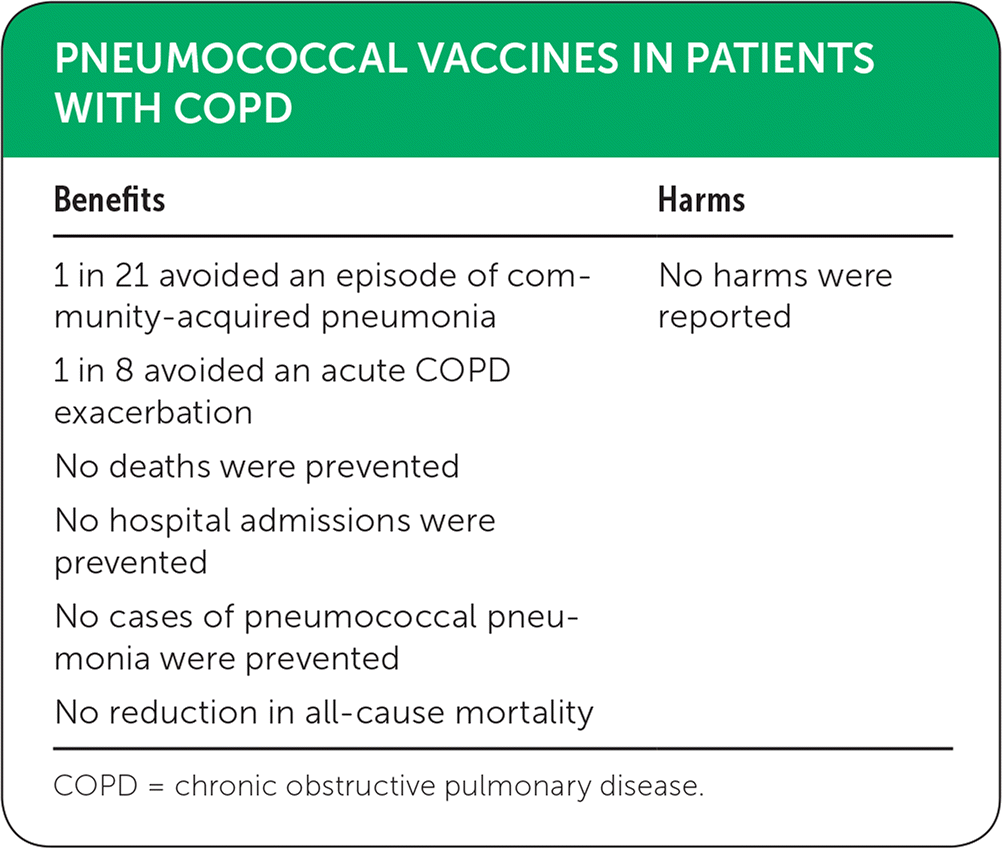
| Benefits | Harms |
|---|---|
| 1 in 21 avoided an episode of community-acquired pneumonia | No harms were reported |
| 1 in 8 avoided an acute COPD exacerbation | |
| No deaths were prevented | |
| No hospital admissions were prevented | |
| No cases of pneumococcal pneumonia were prevented | |
| No reduction in all-cause mortality |
Details for This Review
Study Population: Adults with moderate to severe chronic obstructive pulmonary disease (COPD) without previous pneumococcal vaccination
Efficacy End Points: Incidence of community-acquired pneumonia (CAP), morta lity from cardiorespirator y causes, all-cause mortality, hospital admission, and COPD exacerbation
Harm End Points: Adverse effects of vaccination
Narrative: Worldwide, COPD was the fourth leading cause of death in 2015 with exacerbations of the disease contributing to significant health care costs and decline in patient quality of life.1–4 Infection with Streptococcus pneumoniae is strongly associated with the development of CAP in persons with COPD. However, until recently, the benefit of vaccination against S. pneumoniae for preventing pneumonia was only studied in populations without a diagnosis of COPD.5–7 In this 2017 Cochrane review of 12 randomized controlled trials with 2,171 participants, the effect of pneumococcal vaccination was compared with that of a control or alternative vaccine type.
Pneumococcal vaccines had a significant effect on reducing CAP when compared with control (number needed to treat [NNT] = 21; six randomized controlled trials [RCTs], N = 1,372; odds ratio [OR] = 0.62; 95% confidence interval [CI], 0.43 to 0.89; moderate certainty evidence), but they had no effect on reducing episodes of pneumococcal pneumonia (three RCTs, N = 1,158; OR = 0.26; 95% CI, 0.5 to 1.31; low certainty evidence). A paucity of confirmed cases of pneumococcal pneumonia may contribute to the lack of evidence for pneumococcal vaccines preventing this disease.8
Pneumococcal vaccines had no significant effect on mortality from cardiorespiratory disease when compared with control (three RCTs, N = 888; OR = 1.07; 95% CI, 0.69 to 1.66; moderate certainty evidence) and had no significant effect on all-cause mortality (five RCTs, N = 1,053; OR = 1.00; 95% CI, 0.72 to 1.40; moderate certainty evidence).8
Pneumococcal vaccines reduced the number of COPD exacerbations (NNT = 8; four RCTs, N = 446; OR = 0.60; 95% CI, 0.39 to 0.93; moderate certainty evidence), but did not significantly reduce the number of hospital admissions (three RCTs, N = 391; OR = 0.74; 95% CI, 0.32 to 1.74; moderate certainty evidence).8
Caveats: Three studies did not blind their participants or use a placebo for comparison, leading to a high risk of bias. Most studies were only at moderate risk of bias because of missing information regarding methods of allocation and concealment. No data were reported comparing adverse outcomes with pneumococcal vaccines vs. standard of care. Although there was no improvement in all-cause mortality, the benefit in vaccination lies in the potential cost and manpower savings. Based on 2008 data, there were nearly 17 million outpatient and emergency department visits related to managing COPD at an estimated cost of $42.6 billion.9 Preventing even a modest number of these visits would translate to considerable reduction in the annual health care burden. The Centers for Disease Control and Prevention recommends that anyone with chronic pulmonary disease between 19 and 64 years of age should routinely receive the 23-valent pneumococcal polysaccharide vaccine (PPSV23; Pneumovax 23). At age 65 years, these patients should next receive the 13-valent pneumococcal conjugate vaccine (PCV13; Prevnar 13) if they have not previously received it, and another dose of the PPSV23 at least one year after receiving PCV13 and at least 5 years after receiving PPSV23.10 [corrected]