
Am Fam Physician. 2018;97(9):594-599
Author disclosure: No relevant financial affiliations.
Acute Charcot neuroarthropathy of the foot and ankle is often difficult to diagnose because of limited findings in the patient history, physical examination, imaging, and laboratory studies. Delay in treatment results in the development of rigid foot and ankle deformities, increasing the risk of ulceration, infection, and major lower extremity amputation. Acute Charcot neuroarthropathy should be suspected in any patient 40 years or older with obesity and peripheral neuropathy who presents with an acutely swollen foot following minimal or no recalled trauma and who reports minimal to no pain, particularly if radiography and laboratory markers of infection are normal. Magnetic resonance imaging or computed tomography should be performed in these cases. If changes consistent with acute Charcot neuroarthropathy are observed, prompt immobilization and/or referral to a foot and ankle subspecialist is needed to minimize sequelae. Immobilization should continue until lower extremity edema and warmth resolve, and serial radiography shows evidence of osseous consolidation. Intranasal calcitonin salmon may have a role as adjunctive therapy. Although controversial, surgery may be indicated if there is severe dislocation or instability, concern for skin breakdown, or failure of conservative treatment to obtain a stable, plantigrade foot.
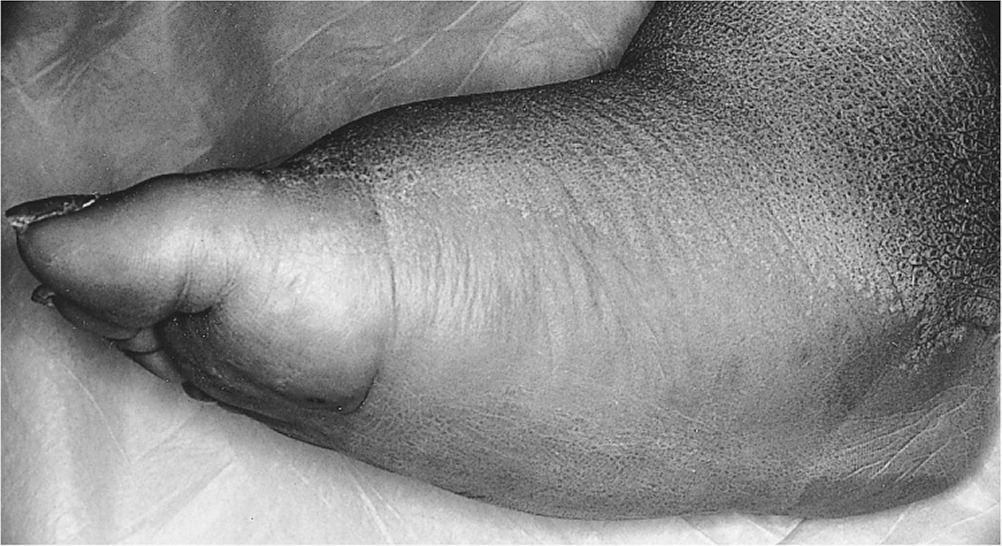
Charcot neuroarthropathy of the foot and ankle is an inflammatory condition affecting the periarticular soft tissues and bone in persons with peripheral neuropathy, resulting in osseous subluxation, dislocation, and fracture, if the lower extremity is not immobilized.1 One in four cases of acute Charcot foot is misdiagnosed, most often as cellulitis, gout, deep venous thrombosis, or a minor sprain2–13 (Table 12–8,14–16), which delays diagnosis by an average of seven months.2,17–19 Without prompt treatment, the condition often results in development of rigid foot deformities (i.e., the classic rocker-bottom foot [Figure 120]), increasing the risk of major lower extremity amputation by 15- to 40-fold.6
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| In patients with suspected acute Charcot neuroarthropathy, early and accurate diagnosis and prompt immobilization reduce the incidence of rigid foot deformity development, which increases patient quality of life and reduces the risk of ulceration, infection, and amputation. | C | 2–6, 8–11, 14, 17, 20–29 |
| The diagnosis of acute Charcot neuroarthropathy should be considered in any patient 40 years or older with obesity and peripheral neuropathy who presents with a unilateral swollen limb and minimal or no associated pain. | C | 2–12, 14, 18–20, 22–25, 29–37 |
| Acute Charcot neuroarthropathy should be considered in patients with recurrent cellulitis but no systemic or laboratory findings concerning for infection. | C | 2–14, 22, 30, 38, 42 |
| Bilateral weight-bearing radiography is recommended to allow for comparison between both feet in persons with suspected acute Charcot neuroarthropathy. Clinicians should look for signs of subtle subluxations or ligamentous avulsion, which denote impeding osseous instability. | C | 2–4, 9–12, 14, 20, 22–26, 30, 38, 39 |
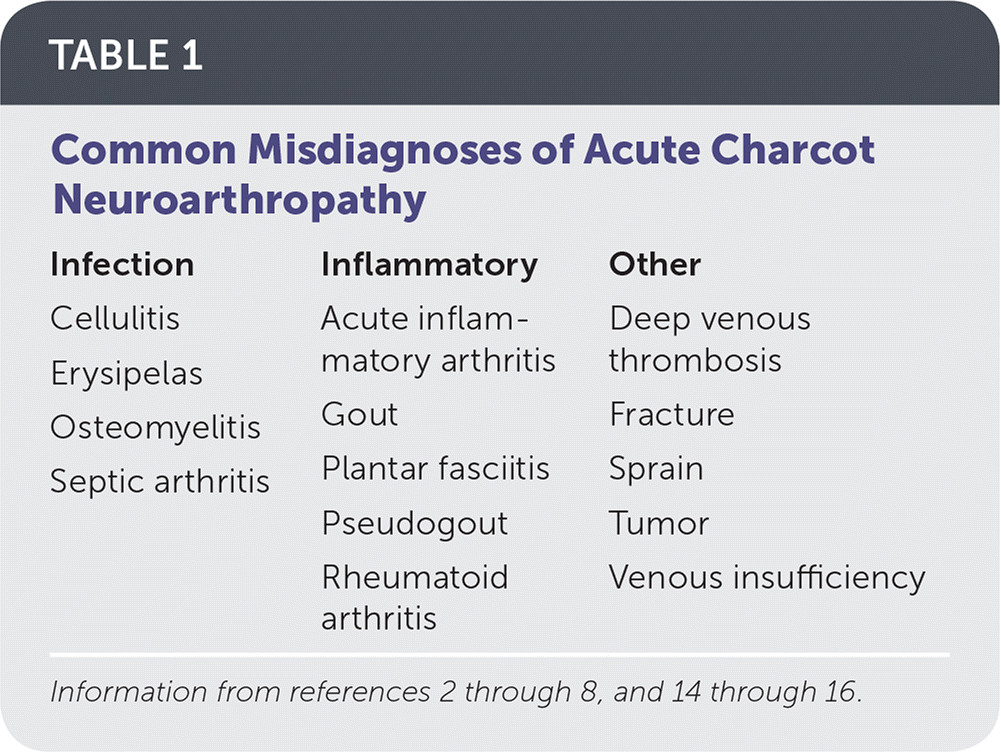
| Infection | Inflammatory | Other |
|---|---|---|
| Cellulitis | Acute inflammatory arthritis | Deep venous thrombosis |
| Erysipelas | Gout | Fracture |
| Osteomyelitis | Plantar fasciitis | Sprain |
| Septic arthritis | Pseudogout | Tumor |
| Rheumatoid arthritis | Venous insufficiency |
This article reviews key elements of the history and physical examination, imaging, and laboratory studies that can increase clinical suspicion for acute Charcot neuroarthropathy in patients experiencing a first episode. Consideration of these elements can assist with early and accurate diagnosis. Prompt initiation of lower extremity immobilization, with referral to a foot and ankle subspecialist as needed, can minimize the sequelae (e.g., rigid foot deformity, ulceration, infection, amputation) and improve patients' quality of life.2–6,8–11,14,17,20–29
History
The diagnosis of acute Charcot neuroarthropathy should be considered in any patient 40 years or older with obesity and peripheral neuropathy who presents with a unilateral swollen limb and minimal or no associated pain.2–12,14,18–20,22–25,29–37 Diabetes mellitus is the most common cause of peripheral neuropathy in the United States. The lifetime prevalence of Charcot neuroarthropathy in patients with diabetes ranges from 0.1% to 10%, increasing to 29% to 35% if peripheral neuropathy is present.2,3,12,24–26,30,38,39 Compared with those who have type 2 diabetes, patients with type 1 diabetes tend to have diabetes for a longer duration (24 ± 8.4 years vs. 13 ± 8.1 years) and be younger (42 ± 10.2 years vs. 59 ± 7.8 years) when acute Charcot neuroarthropathy develops.6,31 Patients with type 1 diabetes also tend to be less obese and more prone to recurrent events of Charcot neuroarthropathy than patients with type 2 diabetes.32 An A1C level greater than 9% is associated with a 30% increased risk of developing Charcot neuroarthropathy.31
Misdiagnosis of acute Charcot neuroarthropathy may occur when it presents in patients who have peripheral neuropathy from etiologies other than diabetes, such as alcoholism, use of chemotherapeutic agents, inherited disorders, or trauma.34 Other factors that increase the risk of acute Charcot neuroarthropathy include a history of foot ulceration; retinopathy; nephropathy, renal failure, or renal transplantation; rheumatoid arthritis; iron deficiency anemia; or obesity.6,12,31 Between 25% and 50% of patients recall no trauma or inciting event, and the same number do not report pain.2,5–10,14,17–20,22,23,39 If an inciting event is recalled, it is often minor, such as walking a long distance or sustaining a minor sprain. Patients who recall an inciting event are more likely to report pain, often described as a constant discomfort not severe enough to stop ambulation.5,9,12
Physical Examination and Laboratory Studies
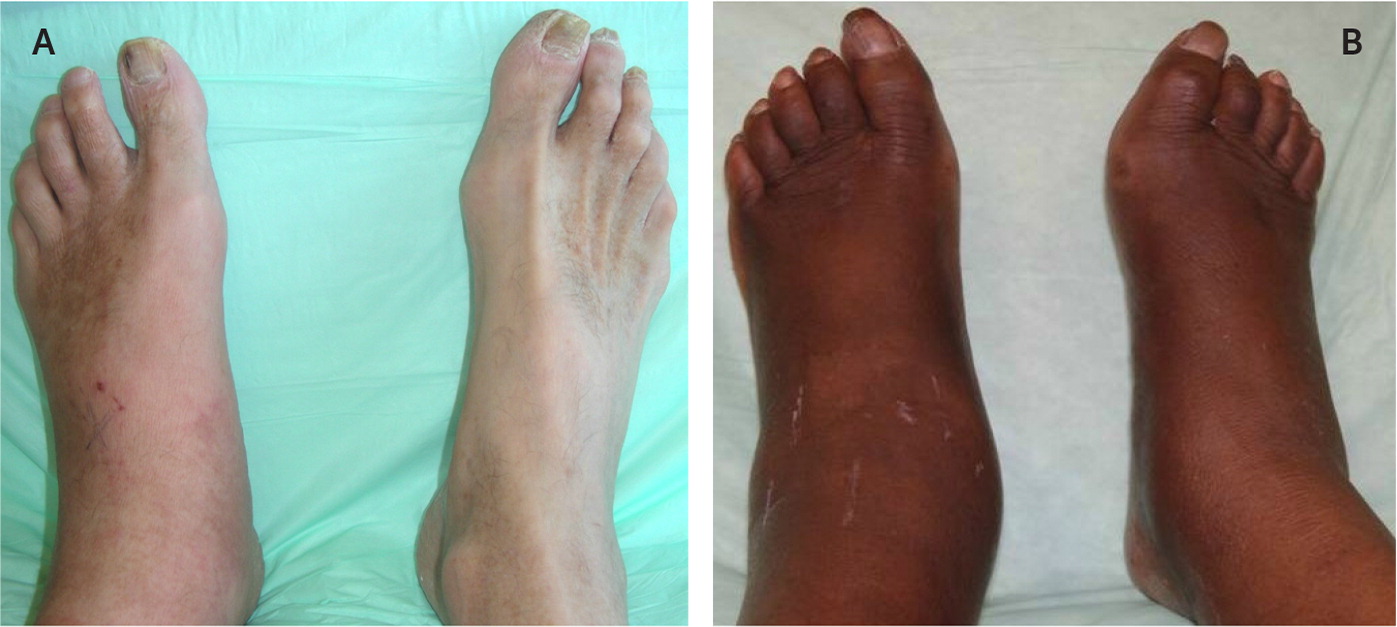
Patients with acute Charcot neuroarthropathy are usually afebrile with normal vital signs. More than 70% of the time, patients have unilateral lower extremity edema (Figure 2) with associated erythema and increased warmth (2°C to 8°C [3.6°F to 14.4°F]) with no open wound.2,4,10,11,14,17,18,20–22,39 Peripheral neuropathy can be confirmed with use of a Semmes-Weinstein monofilament test. Deep tendon reflexes are also severely impaired, particularly the Achilles tendon reflex.6 Lower extremity edema extends down to the foot, which is particularly swollen around the affected joints.14 Pedal pulses are most often palpable and can be described as bounding if unobscured by edema.6 The increase in skin temperature can assist in diagnosis and resolution of the Charcot process.35 Less expensive, industrial-grade, noncontact infrared thermometers have been shown to be as reliable in detecting temperature of the skin as a more expensive medical infrared thermometer.36
Erythema secondary to the inflammation of acute Charcot neuroarthropathy can be differentiated from infection by elevating the affected extremity above the level of the heart for five to 10 minutes. Erythema secondary to acute Charcot neuroarthropathy will dissipate, whereas erythema from infection will not.13 Laboratory markers of infection, such as white blood cell count, C-reactive protein level, and erythrocyte sedimentation rate, are usually normal.11,12,14,17,25,38,40
Patients with these findings may have been previously treated for cellulitis. Acute Charcot foot should be considered in patients with recurrent cellulitis but no systemic or laboratory findings concerning for infection.2–14,22,30,31,38 Antibiotic therapy does not resolve symptoms, which may have led to previous hospital admission. Symptom resolution may occur during hospitalization because patients spend more time in bed; however, symptoms may return after discharge when patients are ambulatory, which may lead to a misdiagnosis of recurrent cellulitis. Although acute Charcot neuroarthropathy can coexist with cellulitis, osteomyelitis, or open wounds of the lower extremity, the latter two are more common with recurrent episodes.
Imaging Studies
VENOUS DUPLEX ULTRASONOGRAPHY
Venous duplex ultrasonography may be performed when deep venous thrombosis is suspected. Results should be normal in acute Charcot neuroarthropathy.
RADIOGRAPHY
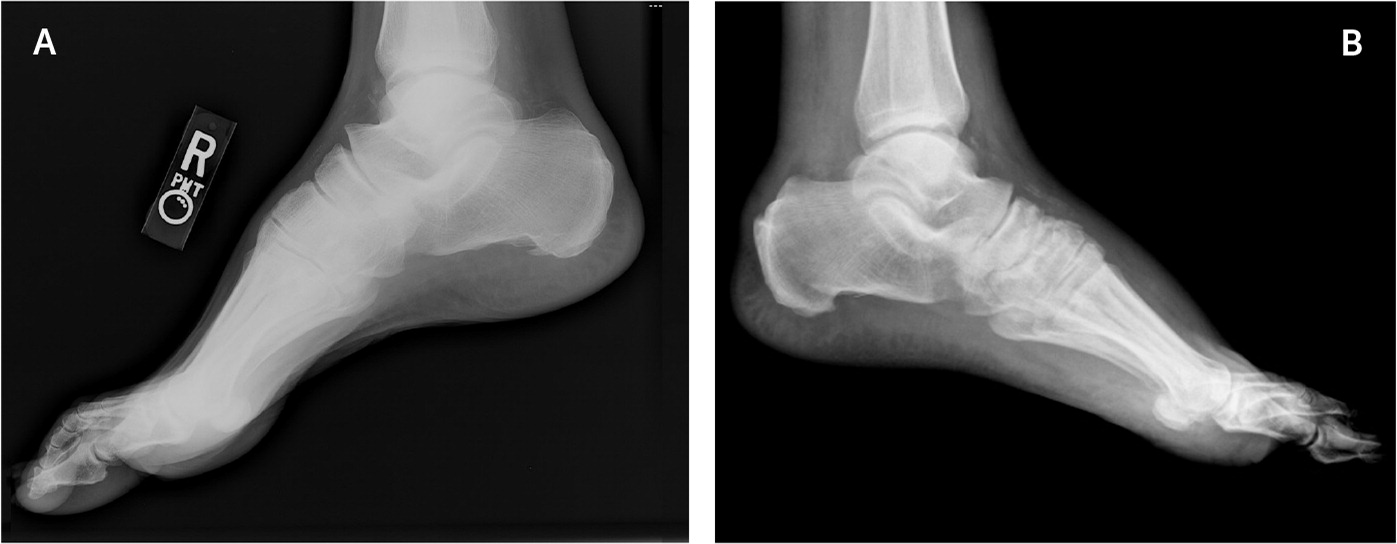
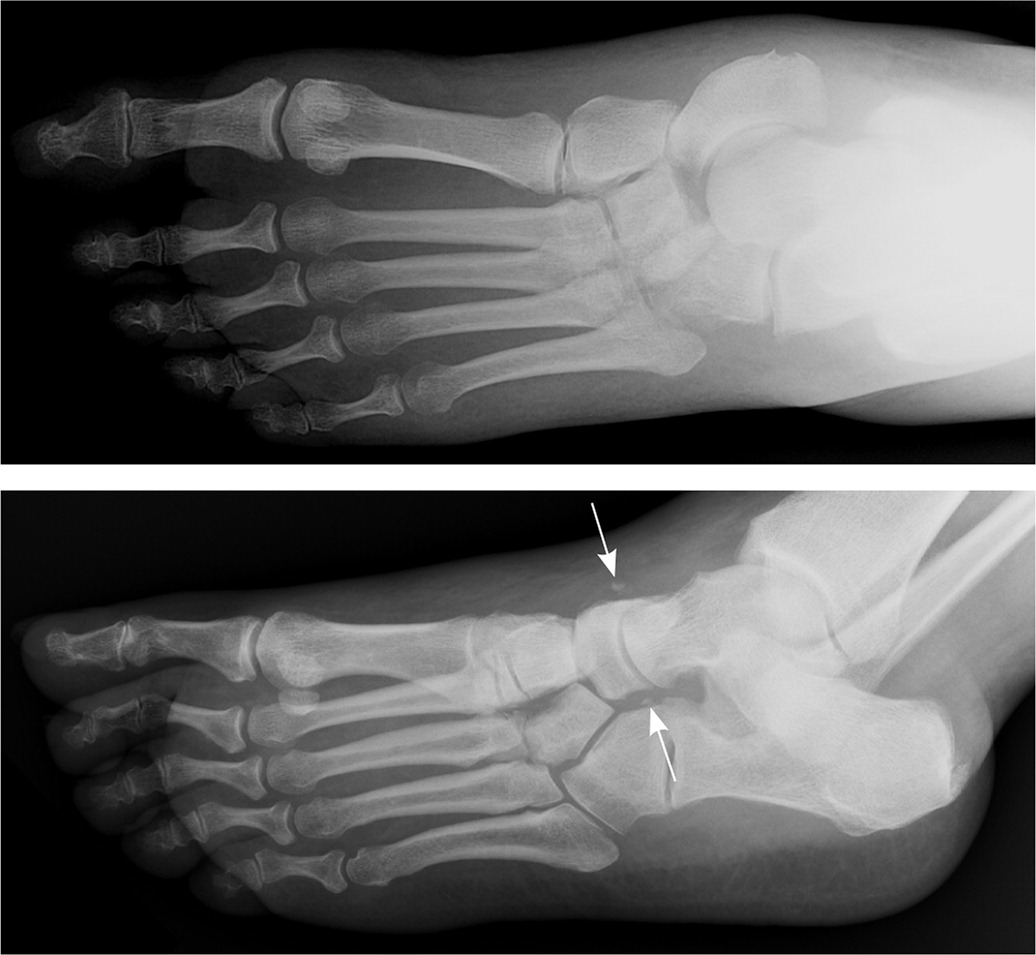
Radiography should be the primary imaging study. Although the characteristic bony destruction of Charcot neuroarthropathy can take six to 12 months to become visible on radiography, initial imaging serves as a baseline and allows for detection of subtle changes denoting instability. Subtle subluxations and small flecks of bone secondary to ligamentous avulsion fractures may be present, signaling instability and impending osseous destruction2–4,9–12,14,17,20–26,30,38,39 (Figures 3 and 4). More than 60% of all Charcot neuroarthropathy events affect the midfoot.12,24 Osseous overlap in the mid-foot can make subtle subluxations and minimally displaced fractures difficult to visualize.41–43 For this reason, bilateral weight-bearing radiography is recommended for comparison of both feet.2,3,17,20,21
MAGNETIC RESONANCE IMAGING
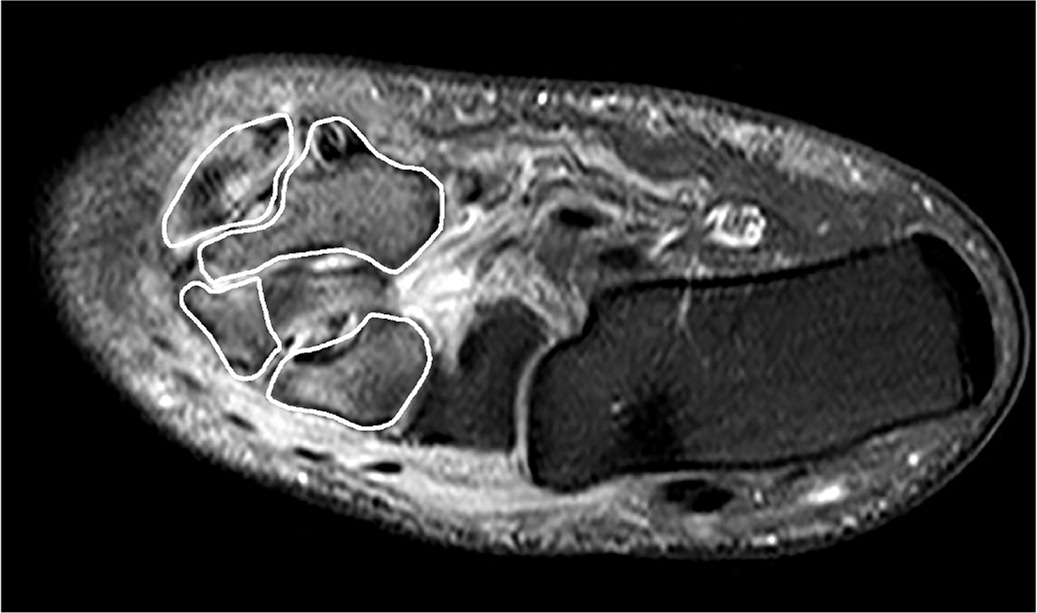
Magnetic resonance imaging (MRI) in the acute phase of Charcot neuroarthropathy can demonstrate periarticular bone marrow edema (Figure 5), adjacent soft tissue edema, joint effusion, and microtrabecular or stress fractures when radiography results are normal.5,24,33,37,44,45 A study that followed 71 cases (59 patients) of acute Charcot neuroarthropathy over 12 years concluded that radiography was insufficient in diagnosing midfoot fractures; that any bone marrow edema present on MRI was likely to progress to cortical fracture if unprotected ambulation was allowed; and that cortical fractures were a significant marker for impending deformit y development.33 Performing MRI is recommended when radiography results are inconclusive and patients exhibit key clinical elements outlined in Table 2.
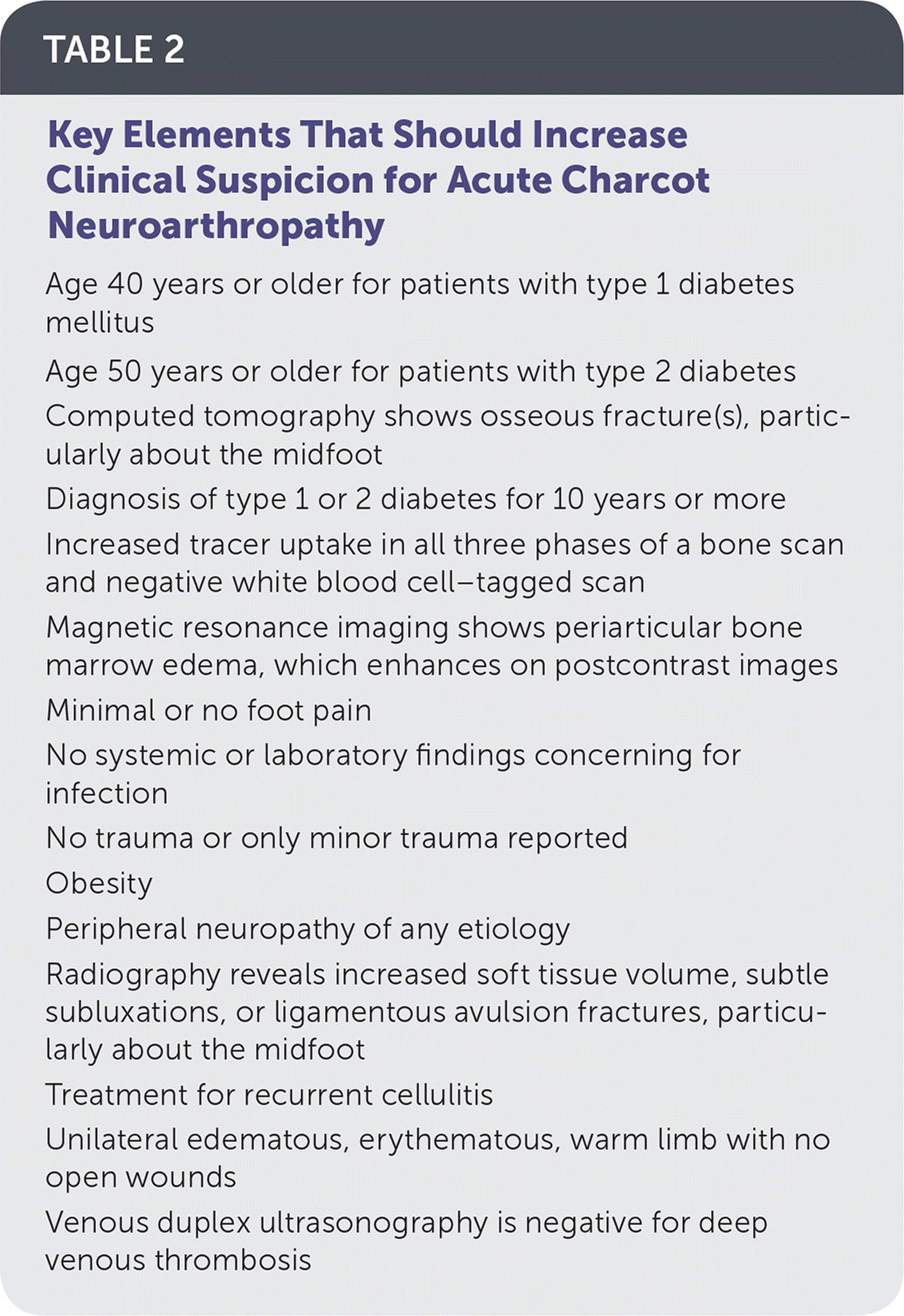
| Age 40 years or older for patients with type 1 diabetes mellitus |
| Age 50 years or older for patients with type 2 diabetes |
| Computed tomography shows osseous fracture(s), particularly about the midfoot |
| Diagnosis of type 1 or 2 diabetes for 10 years or more |
| Increased tracer uptake in all three phases of a bone scan and negative white blood cell–tagged scan |
| Magnetic resonance imaging shows periarticular bone marrow edema, which enhances on postcontrast images |
| Minimal or no foot pain |
| No systemic or laboratory findings concerning for infection |
| No trauma or only minor trauma reported |
| Obesity |
| Peripheral neuropathy of any etiology |
| Radiography reveals increased soft tissue volume, subtle subluxations, or ligamentous avulsion fractures, particularly about the midfoot |
| Treatment for recurrent cellulitis |
| Unilateral edematous, erythematous, warm limb with no open wounds |
| Venous duplex ultrasonography is negative for deep venous thrombosis |
COMPUTED TOMOGRAPHY
BONE SCANS
Increased tracer uptake to the affected joint occurs in all three phases of a bone scan when acute Charcot neuroarthropathy is present. White blood cell–tagged scans are often negative.4,12 There is limited literature available on the aid of nuclear bone scanning in the diagnosis of acute Charcot neuroarthropathy. Because of the nonspecific nature of the study, radiography and MRI are recommended over nuclear medicine studies.
Treatment
IMMOBILIZATION
The mainstay of treatment of Charcot neuroarthropathy is immobilization in a total contact cast, which increases the total surface area of contact to the entire lower extremity, distributing pressure away from the foot. Immobilization should continue until lower extremity edema and warmth resolve and serial radiography shows evidence of osseous consolidation, which typically occurs after three to four months but can take up to 12 months.2–5,10,13,14,17,20,21,26–30,33
Computed tomography and MRI may also be used to determine resolution. Bone scanning is not recommended for determining resolution because osseous remodeling can continue for up to one year, resulting in prolonged increased uptake in the affected area due to remodeling. Removable walking boots and braces that incorporate the entire lower leg have also been used and are better tolerated by patients than cast immobilization, in addition to reducing the risk of iatrogenic complications that can occur with casting.28,38 However, these removable devices require a longer duration of immobilization.18,23,28
SURGERY
BISPHOSPHONATES
A systematic review of 10 small heterogeneous studies concluded that the adjunctive use of bisphosphonates during conservative management of acute Charcot neuroarthropathy was not effective.49
INTRANASAL CALCITONIN
A single randomized controlled trial of 32 patients with acute Charcot neuroarthropathy who were treated with cast immobilization and oral calcium supplementation compared 200 IU of intranasal calcitonin salmon daily with no adjunctive therapy. A significantly greater reduction in markers of bone turnover in the intervention group was noted at three and six months, suggesting that daily intra-nasal calcitonin may be an effective adjunctive treatment.50 However, no studies of calcitonin have reported patient-oriented outcomes.
This article updates previous articles on this topic by Sommer and Lee,20 and Caputo, et al.14
Data Sources: A PubMed search was performed of peer-reviewed journals, restricted to the English language with no restriction on date, using the key words acute, stage 0, Charcot, and Charcot neuroarthropathy. Each reference was manually searched for additional pertinent references. This review included studies of all levels of evidence. We also searched the Agency for Healthcare Research and Quality evidence reports, U.S. Preventive Services Task Force, Cochrane Database of Systematic Reviews, Clinical Evidence, evidence-based guidelines from the National Guideline Clearinghouse, and the Institute for Clinical Systems Improvement. Search date: April 10, 2017.
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or reflecting the views of the Department of the Army or the Department of Defense.
