
Am Fam Physician. 2018;97(11):715-716
Author disclosure: No relevant financial affiliations.
Clinical Question
Does the atypical antipsychotic risperidone (Risperdal) safely and effectively treat disruptive behavior disorders in children and adolescents?
Evidence-Based Answer
Risperidone reduces measures of aggression and improves conduct in children with disruptive behavior disorders; however, only short-term use is recommended. Weight gain of 2 to 2.5 kg (4.4 to 5.5 lb) is common. There is insufficient evidence to evaluate the benefits of other antipsychotics.1 (Strength of Recommendation: A, based on consistent, good-quality patient-oriented evidence.)
Practice Pointers
Disruptive behavior disorders in children and adolescents include conduct disorder and oppositional defiant disorder. These disorders are common, affecting 5.7% of children.2 The authors of this Cochrane review sought to demonstrate whether atypical antipsychotics safely and effectively reduce aggression and improve conduct in children and adolescents with these disorders.1
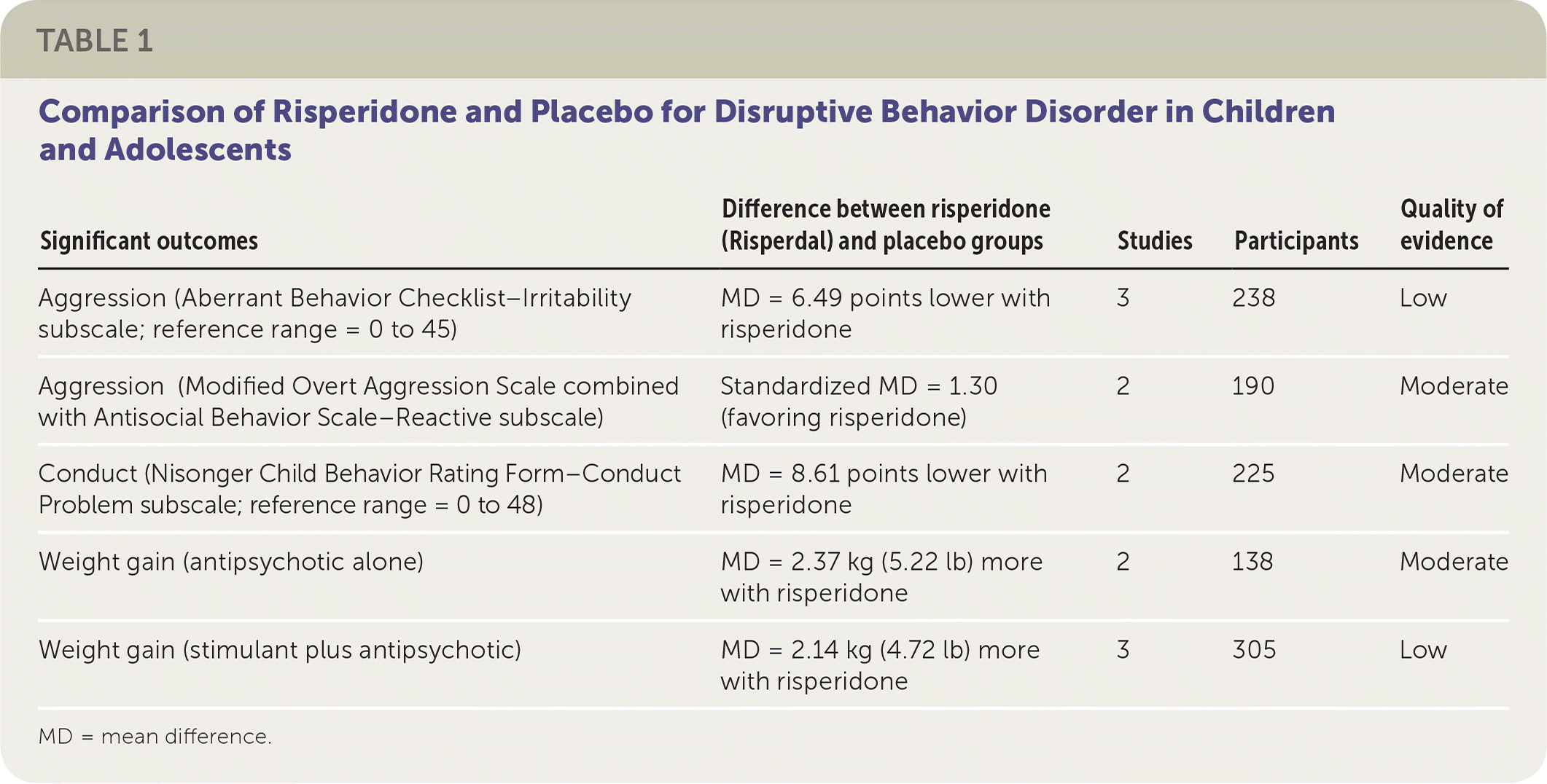
| Significant outcomes | Difference between risperidone (Risperdal) and placebo groups | Studies | Participants | Quality of evidence |
|---|---|---|---|---|
| Aggression (Aberrant Behavior Checklist–Irritability subscale; reference range = 0 to 45) | MD = 6.49 points lower with risperidone | 3 | 238 | Low |
| Aggression (Modified Overt Aggression Scale combined with Antisocial Behavior Scale–Reactive subscale) | Standardized MD = 1.30 (favoring risperidone) | 2 | 190 | Moderate |
| Conduct (Nisonger Child Behavior Rating Form–Conduct Problem subscale; reference range = 0 to 48) | MD = 8.61 points lower with risperidone | 2 | 225 | Moderate |
| Weight gain (antipsychotic alone) | MD = 2.37 kg (5.22 lb) more with risperidone | 2 | 138 | Moderate |
| Weight gain (stimulant plus antipsychotic) | MD = 2.14 kg (4.72 lb) more with risperidone | 3 | 305 | Low |
Three trials using risperidone measured aggression with the Aberrant Behavior Checklist–Irritability subscale (reference range: 0 to 45). Patients taking risperidone scored, on average, 6.49 points lower than those taking placebo (95% confidence interval [CI], −8.79 to −4.19). One risperidone trial used the Modified Overt Aggression Scale, whereas another used the two-part Antisocial Behavior Scale. Both parts of the Antisocial Behavior Scale were analyzed separately with the trial that used the Modified Overt Aggression Scale. When the Antisocial Behavior Scale–Reactive subscale was combined with the Modified Overt Aggression Scale, the analysis showed significant improvement after risperidone therapy. This change, a standardized mean difference of −1.30 (95% CI, −2.21 to −0.40), is considered clinically significant.
Conduct was measured via the Nisonger Child Behavior Rating Form–Conduct Problem subscale (reference range: 0 to 48). In a meta-analysis of two trials, patients treated with risperidone scored on average 8.61 points lower than those in the placebo group (95% CI, −11.49 to −5.74). This result is also considered clinically significant.
The most commonly reported adverse effect was weight gain. Patients taking risperidone alone gained an average of 2.37 kg (5.22 lb) more than patients taking placebo (95% CI, 0.26 to 4.49), whereas patients taking both a stimulant and risperidone gained an average of 2.14 kg (4.72 lb) more than those taking placebo (95% CI, 1.04 to 3.23). Metabolic laboratory changes were reported in one trial that involved 168 children.3 It showed a significant incidence of hyperprolactinemia in the risperidone group (68% vs. 5% with placebo); however, only one patient taking risperidone had a clinically significant prolactin elevation.
Guidelines from the National Institute for Health and Care Excellence recommend against the routine use of psychotropic medications for disruptive behavior disorders in children and adolescents, but recommend considering short-term risperidone use for explosive anger and severe emotional dysregulation that has been unresponsive to psychosocial interventions.4 Canadian guidelines give a conditional recommendation in favor of risperidone use for disruptive behavior disorders.5
The practice recommendations in this activity are available at http://www.cochrane.org/CD008559.
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the U.S. government.
