
Am Fam Physician. 2018;97(12):808-810
Oxymetazoline hydrochloride 1% cream (Rhofade) is an alpha1A adrenoceptor agonist labeled for the topical treatment of persistent facial erythema associated with rosacea in adults. It is thought to work through topical vasoconstriction.1
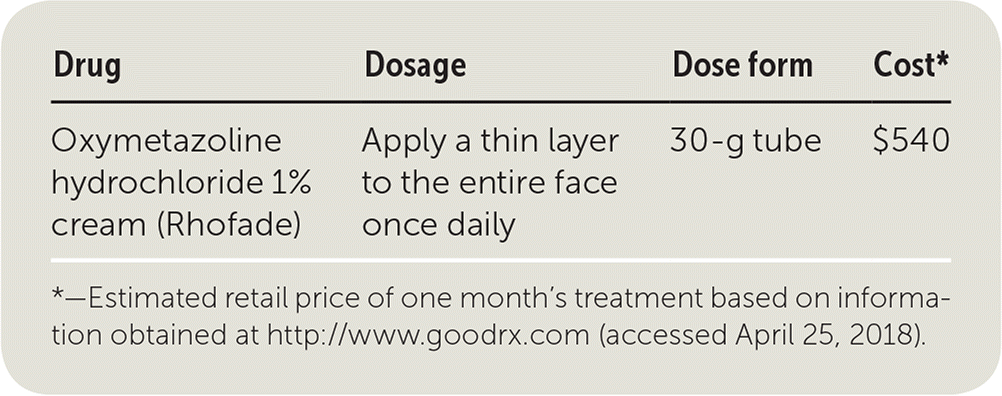
| Drug | Dosage | Dose form | Cost* |
|---|---|---|---|
| Oxymetazoline hydrochloride 1% cream (Rhofade) | Apply a thin layer to the entire face once daily | 30-g tube | $540 |
Safety
Studies have shown oxymetazoline cream to be safe with few adverse effects. Basal cell carcinoma is the most serious adverse effect reported at a rate of 1.3%, although this rate does not appear to be higher than at baseline.2,3 Systemic alpha-adrenergic effects are possible, but they have not been reported in clinical studies. Oxymetazoline cream has not been evaluated in patients with vascular insufficiency, orthostatic hypotension, cardiovascular disease, or narrow-angle glaucoma. It has not been evaluated in pregnant or breastfeeding women, but risk of fetal or infant harm is not expected because of minimal systemic absorption.
Tolerability
Oxymetazoline cream is generally well tolerated. Application site dermatitis, pruritus, worsening erythema, and pain will occur in 1% to 3% of users. In two studies, 2.6% of patients discontinued treatment compared with 0.5% of patients using placebo cream.1
Effectiveness
Oxymetazoline cream will improve redness scores by at least two points on a five-point scale by clinical assessment in 12% to 18% of patients with moderate to severe rosacea (number needed to treat [NNT] = 17) after one month of treatment. Patient assessment is somewhat more favorable, with 24% of patients reporting an improvement of at least two points (NNT = 12). These results were demonstrated in two clinical trials of 884 patients comparing oxymetazoline cream with vehicle cream.2–5 Oxymetazoline cream has not been directly compared with other treatments for rosacea and has not been studied in combination with other treatments.
Price
A one-month supply of oxymetazoline cream (one 30-g tube) costs approximately $540. In comparison, one 45-g tube of metronidazole 0.75% cream costs approximately $83. Brimonidine topical gel is similar in price to oxymetazoline cream, costing about $440 for a 30-g tube. A case report of two patients that used oxymetazoline 0.05% nasal spray off-label for rosacea showed it to be effective with good tolerability. This is a much less expensive option, costing approximately $2 for a 1-oz bottle.6
Simplicity
Oxymetazoline cream should be applied once daily in a thin layer covering the entire face, avoiding eyes and lips. Patients should be instructed to wash their hands after application.1
Bottom Line
Oxymetazoline cream has been shown to be safe and effective at reducing erythema in a small portion of treated patients, but research showing its effectiveness relative to other options is lacking. It is more expensive than alternative treatment options and should be considered in patients for whom other treatments have not decreased erythema sufficiently. It has not been studied in patients with cardiovascular conditions.7
