Am Fam Physician. 2018;98(4):246-247
Author disclosure: No relevant financial affiliations.
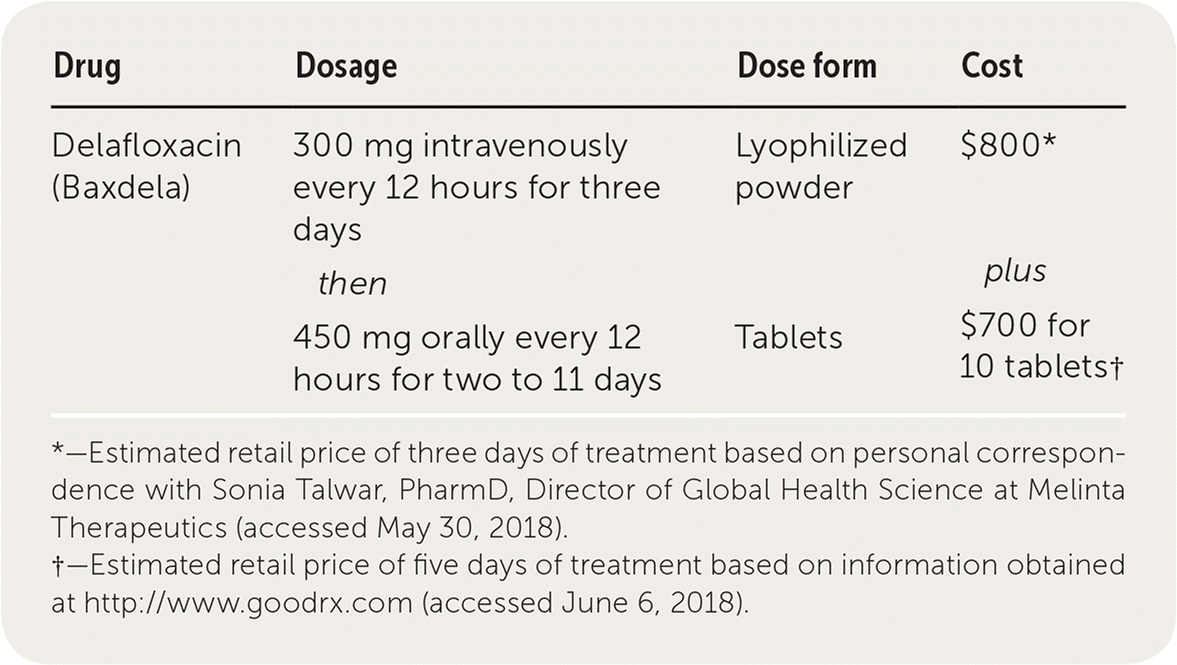
Delafloxacin (Baxdela) is a fluoroquinolone antibiotic labeled for the treatment of adults with serious acute bacterial skin and skin structure infections, including those caused by Staphylococcus aureus (methicillin-sensitive and methicillin-resistant), Enterococcus faecalis, and Pseudomonas aeruginosa.1 Although delafloxacin is approved by the U.S. Food and Drug Administration for a strictly oral regimen, patients enrolled in the clinical trials that led to approval required three days of initial intravenous therapy followed by oral therapy.1
Safety
As with all fluoroquinolones, the most significant risks of delafloxacin therapy include rare but serious tendinitis/tendon rupture, peripheral neuropathy, and central nervous system adverse effects.1 Treatment should be discontinued if any of these reactions occur. As with other antimicrobials, delafloxacin can cause Clostridium difficile–associated diarrhea. Delafloxacin has not been studied in pregnant or breastfeeding women, and it should not be used in patients younger than 18 years.1
Tolerability
Delafloxacin was well-tolerated by most patients in clinical trials. Some patients (8%) experienced nausea and/or vomiting.1 In premarketing studies, the discontinuation rate was 0.9% for patients taking delafloxacin vs. 2.8% for patients in the comparator groups. The most commonly reported reasons for discontinuation were urticaria (0.3%) and hypersensitivity (0.3%).1
Effectiveness
Delafloxacin has been studied in two trials that enrolled adults with cellulitis/erysipelas, wound infection, major cutaneous abscess, or burn infection requiring initial intravenous therapy, followed by either oral or intravenous treatment for an additional two to 11 days. Methicillin-resistant S. aureus (MRSA) was cultured from approximately 25% of the wound cultures and P. aeruginosa was present in 2% of cultures. Delafloxacin was shown to produce complete or near complete resolution of signs and symptoms at 14 days in about 82% of patients who received intravenous therapy for the full duration and in about 87% of patients who received three days of intravenous treatment followed by oral therapy. The success rates of treatment with either delafloxacin regimen were similar to those achieved in the comparator groups in both studies, which included treatment with a combination of vancomycin and aztreonam (Azactam) administered intravenously for a duration of five to 14 days.1–3
Delafloxacin has not been studied in treating less severe skin and skin structure infections, other infections including those of the urinary or respiratory tracts, or without initial intravenous therapy before oral treatment.
Price
The cost of the initial three days of intravenous therapy with delafloxacin is $800. Continuation of intravenous therapy for two to 11 more days ranges from $530 to $2,900, and switching to oral therapy for two to 11 additional days ranges from $280 to $1,530. The cost of an oral-only treatment regimen (without initial intravenous therapy) for five to 14 days ranges from $700 to $2,100.
Simplicity
Delafloxacin is administered every 12 hours. The intravenous dose should be reduced in patients with severe renal dysfunction (glomerular filtration rate of 15 to 29 mL per minute per 1.73 m2). It should not be used in patients with end-stage renal disease or in patients undergoing hemodialysis.1 The oral dose should not be taken with antacids or multivitamins that include iron or zinc, because these may impair absorption.1
Bottom Line
Delafloxacin is a new fluoroquinolone antibiotic available in intravenous and oral formulations for the treatment of serious skin and skin structure infections in patients requiring initial intravenous therapy. It is effective for infections caused by MRSA or P. aeruginosa, in addition to other common causative pathogens, but has not been shown to be more effective than existing antibiotic regimens. Until delafloxacin is studied further, it should not be used to treat skin and skin structure infections in nonhospitalized patients or for other sites of infection.