Am Fam Physician. 2018;98(5):319-322
Related Practice Guideline: Treatment of Chronic Insomnia in Adults: ACP Guideline.
Author disclosure: No relevant financial affiliations.
Key Clinical Issue
What are the effectiveness, comparative effectiveness, and adverse effects of interventions for insomnia disorder in adults?
Evidence-Based Answer
Cognitive behavior therapy (CBT) for insomnia improves sleep outcomes in the general adult population. (Strength of Recommendation [SOR]: A, based on consistent, good-quality patient-oriented evidence.) The effectiveness of CBT for insomnia was consistent across different delivery modes (i.e., in person as an individual or with a group, by telephone, through the web, or using a self-help book) and was sustained in the long term, which was defined as at least six months. (SOR: B, based on inconsistent or limited-quality patient-oriented evidence.) There was insufficient evidence to report on the adverse effects of CBT for insomnia. Of the U.S. Food and Drug Administration–approved prescription drugs for insomnia, eszopiclone, zolpidem, and suvorexant improved some outcomes among the general adult population in primarily short-term studies of three months or less. (SOR: A, based on consistent, good-quality patient-oriented evidence.) There was limited evidence for the long-term safety of pharmacotherapy for insomnia (eTable A), although observational studies suggest possible associations with head injuries, cancer, and dementia. Data were insufficient to evaluate the effectiveness of benzodiazepines or over-the-counter sleep aids such as diphenhydramine, doxylamine, or melatonin.1
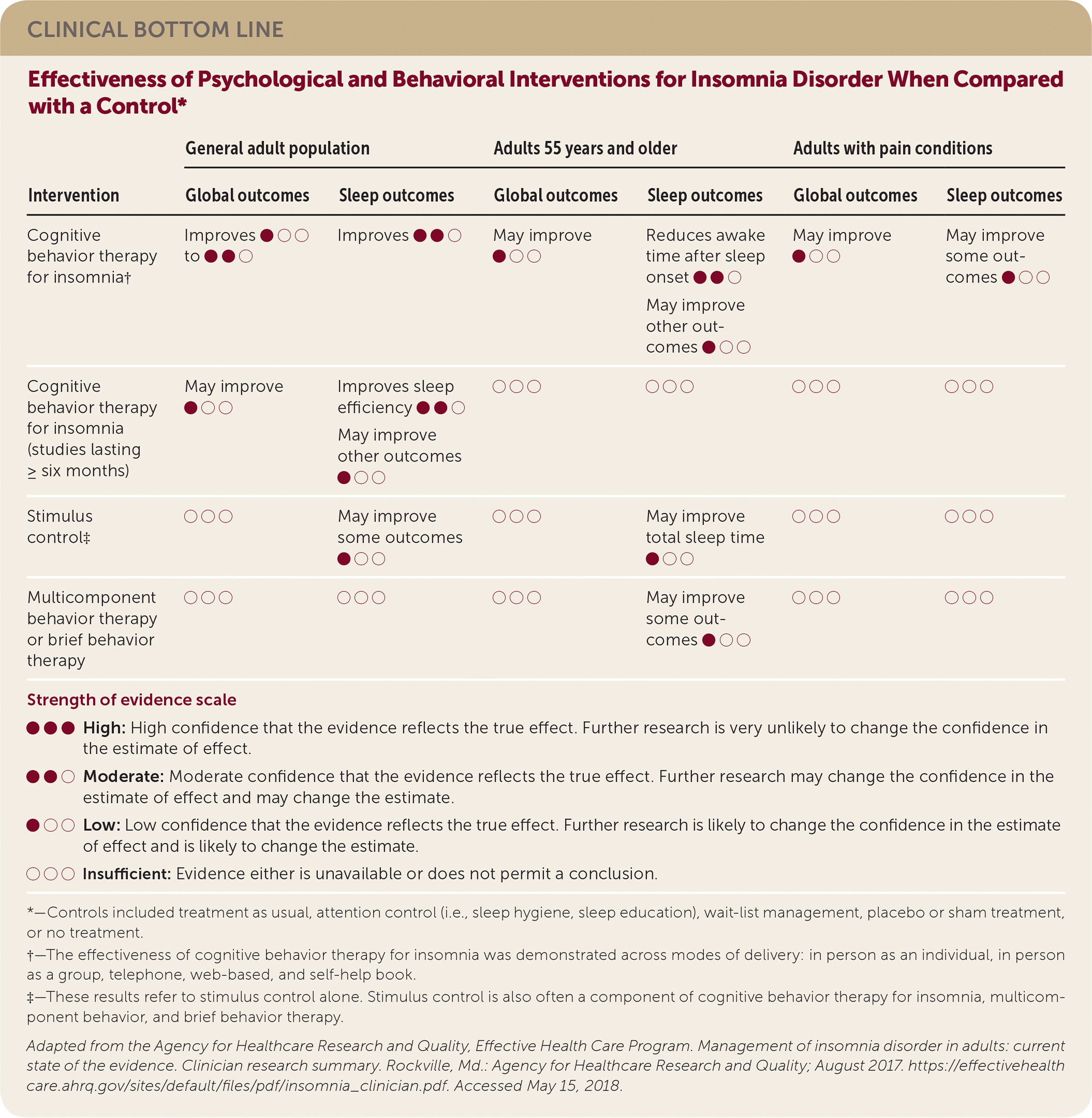
| Intervention | General adult population | Adults 55 years and older | Adults with pain conditions | |||
|---|---|---|---|---|---|---|
| Global outcomes | Sleep outcomes | Global outcomes | Sleep outcomes | Global outcomes | Sleep outcomes | |
| Cognitive behavior therapy for insomnia† | Improves ● ○ ○ to ● ● ○ | Improves ● ● ○ | May improve ● ○ ○ | Reduces awake time after sleep onset ● ● ○ | May improve ● ○ ○ | May improve some outcomes ● ○ ○ |
| May improve other outcomes ● ○ ○ | ||||||
| Cognitive behavior therapy for insomnia (studies lasting ≥ six months) | May improve ● ○ ○ | Improves sleep efficiency ● ● ○ | ○ ○ ○ | ○ ○ ○ | ○ ○ ○ | ○ ○ ○ |
| May improve other outcomes ● ○ ○ | ||||||
| Stimulus control‡ | ○ ○ ○ | May improve some outcomes ● ○ ○ | ○ ○ ○ | May improve total sleep time ● ○ ○ | ○ ○ ○ | ○ ○ ○ |
| Multicomponent behavior therapy or brief behavior therapy | ○ ○ ○ | ○ ○ ○ | ○ ○ ○ | May improve some outcomes ● ○ ○ | ○ ○ ○ | ○ ○ ○ |
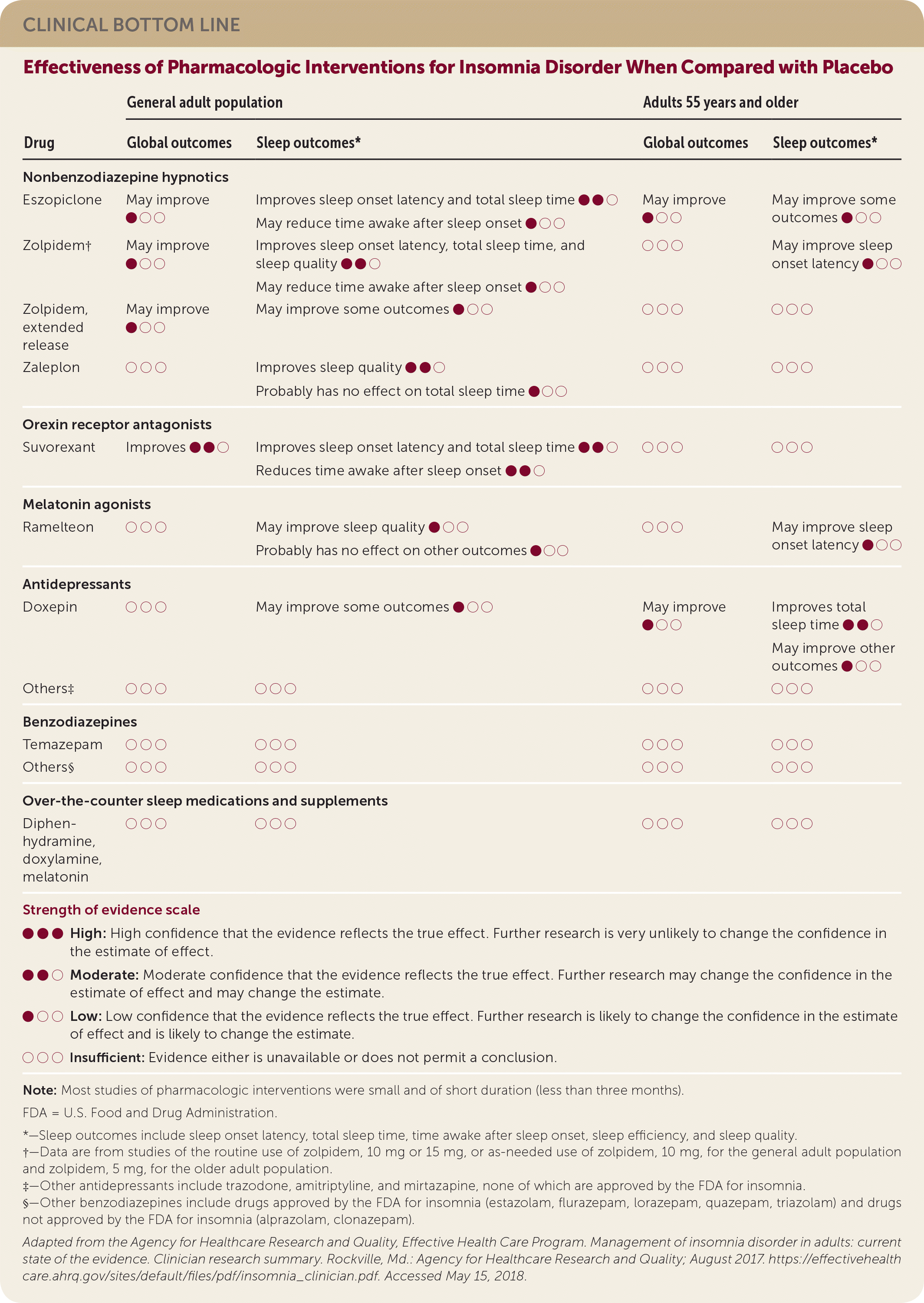
| Drug | General adult population | Adults 55 years and older | ||
|---|---|---|---|---|
| Global outcomes | Sleep outcomes* | Global outcomes | Sleep outcomes* | |
| Nonbenzodiazepine hypnotics | ||||
| Eszopiclone | May improve ● ○ ○ | Improves sleep onset latency and total sleep time ● ● ○ | May improve ● ○ ○ | May improve some outcomes ● ○ ○ |
| May reduce time awake after sleep onset ● ○ ○ | ||||
| Zolpidem† | May improve ● ○ ○ | Improves sleep onset latency, total sleep time, and sleep quality ● ● ○ | ○ ○ ○ | May improve sleep onset latency ● ○ ○ |
| May reduce time awake after sleep onset ● ○ ○ | ||||
| Zolpidem, extended release | May improve ● ○ ○ | May improve some outcomes ● ○ ○ | ○ ○ ○ | ○ ○ ○ |
| Zaleplon | ○ ○ ○ | Improves sleep quality ● ● ○ | ○ ○ ○ | ○ ○ ○ |
| Probably has no effect on total sleep time ● ○ ○ | ||||
| Orexin receptor antagonists | ||||
| Suvorexant | Improves ● ● ○ | Improves sleep onset latency and total sleep time ● ● ○ | ○ ○ ○ | ○ ○ ○ |
| Reduces time awake after sleep onset ● ● ○ | ||||
| Melatonin agonists | ||||
| Ramelteon | ○ ○ ○ | May improve sleep quality ● ○ ○ | ○ ○ ○ | May improve sleep onset latency ● ○ ○ |
| Probably has no effect on other outcomes ● ○ ○ | ||||
| Antidepressants | ||||
| Doxepin | ○ ○ ○ | May improve some outcomes ● ○ ○ | May improve ● ○ ○ | Improves totalsleep time ● ● ○ May improve other outcomes ● ○ ○ |
| Others‡ | ○ ○ ○ | ○ ○ ○ | ○ ○ ○ | ○ ○ ○ |
| Benzodiazepines | ||||
| Temazepam | ○ ○ ○ | ○ ○ ○ | ○ ○ ○ | ○ ○ ○ |
| Others§ | ○ ○ ○ | ○ ○ ○ | ○ ○ ○ | ○ ○ ○ |
| Over-the-counter sleep medications and supplements | ||||
| Diphenhydramine, doxylamine, melatonin | ○ ○ ○ | ○ ○ ○ | ○ ○ ○ | ○ ○ ○ |
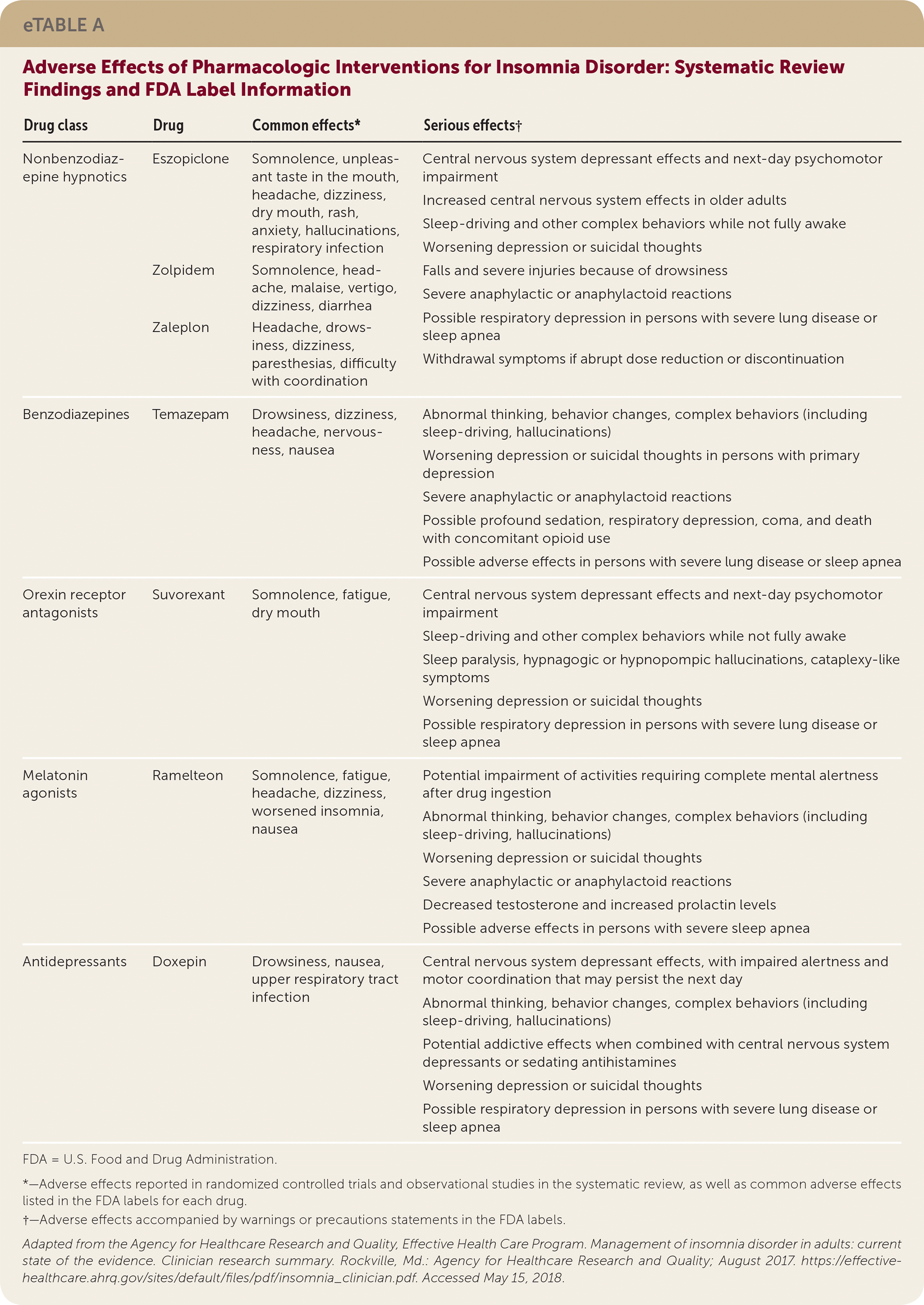
| Drug class | Drug | Common effects* | Serious effects† |
|---|---|---|---|
| Nonbenzodiazepine hypnotics | Eszopiclone | Somnolence, unpleasant taste in the mouth, headache, dizziness, dry mouth, rash, anxiety, hallucinations, respiratory infection | Central nervous system depressant effects and next-day psychomotor impairment Increased central nervous system effects in older adults Sleep-driving and other complex behaviors while not fully awake Worsening depression or suicidal thoughts Falls and severe injuries because of drowsiness Severe anaphylactic or anaphylactoid reactions Possible respiratory depression in persons with severe lung disease or sleep apnea Withdrawal symptoms if abrupt dose reduction or discontinuation |
| Zolpidem | Somnolence, headache, malaise, vertigo, dizziness, diarrhea | ||
| Zaleplon | Headache, drowsiness, dizziness, paresthesias, difficulty with coordination | ||
| Benzodiazepines | Temazepam | Drowsiness, dizziness, headache, nervousness, nausea | Abnormal thinking, behavior changes, complex behaviors (including sleep-driving, hallucinations) Worsening depression or suicidal thoughts in persons with primary depression Severe anaphylactic or anaphylactoid reactions Possible profound sedation, respiratory depression, coma, and death with concomitant opioid use Possible adverse effects in persons with severe lung disease or sleep apnea |
| Orexin receptor antagonists | Suvorexant | Somnolence, fatigue, dry mouth | Central nervous system depressant effects and next-day psychomotor impairment Sleep-driving and other complex behaviors while not fully awake Sleep paralysis, hypnagogic or hypnopompic hallucinations, cataplexy-like symptoms Worsening depression or suicidal thoughts Possible respiratory depression in persons with severe lung disease or sleep apnea |
| Melatonin agonists | Ramelteon | Somnolence, fatigue, headache, dizziness, worsened insomnia, nausea | Potential impairment of activities requiring complete mental alertness after drug ingestion Abnormal thinking, behavior changes, complex behaviors (including sleep-driving, hallucinations) Worsening depression or suicidal thoughts Severe anaphylactic or anaphylactoid reactions Decreased testosterone and increased prolactin levels Possible adverse effects in persons with severe sleep apnea |
| Antidepressants | Doxepin | Drowsiness, nausea, upper respiratory tract infection | Central nervous system depressant effects, with impaired alertness and motor coordination that may persist the next day Abnormal thinking, behavior changes, complex behaviors (including sleep-driving, hallucinations) Potential addictive effects when combined with central nervous system depressants or sedating antihistamines Worsening depression or suicidal thoughts Possible respiratory depression in persons with severe lung disease or sleep apnea |
Practice Pointers
Insomnia is a common problem addressed by family physicians and accounts for more than 5.5 million outpatient visits annually.2 The Diagnostic and Statistical Manual of Mental Disorders, 5th ed., defines insomnia disorder as recurrent poor sleep quality or quantity that causes distress or dysfunction and lasts for longer than three months.3 Insomnia is associated with older age; female sex; mental illness; poor general health; lower socioeconomic status; and decreased memory, mood, and cognitive function.4 Persons with sleep problems report higher levels of depressed mood, anxiety, physical pain and discomfort, and cognitive deficiencies.5
This Agency for Healthcare Research and Quality (AHRQ) review included 59 randomized controlled trials studying psychological interventions in the general adult population, older adults, and adults with pain conditions. Results included global outcomes, such as improvements in sleep and daytime dysfunction or distress, and sleep outcomes, which assessed specific sleep parameters (sleep onset latency, time awake after sleep onset, total sleep time, sleep quality, and sleep efficiency). Duration of insomnia ranged from six months to 19 years, with a duration of more than 10 years in most studies.
Psychological interventions included stimulus control, sleep restriction, relaxation techniques, sleep hygiene education, and CBT for insomnia. CBT for insomnia is a combination of cognitive therapy, behavioral interventions (i.e., sleep restriction and stimulus control), and education (i.e., sleep hygiene). There were insufficient data to draw conclusions on the effectiveness of specific interventions alone (e.g., stimulus control, sleep restriction, relaxation techniques), but based on a meta-analysis of 20 trials, CBT for insomnia improved global and sleep outcomes in the general adult population. Improvements were sustained for at least six months. Among older adults and patients with pain conditions, CBT for insomnia improved global outcomes and some sleep outcomes. There was insufficient evidence to assess adverse effects of psychological treatments.
There were 38 randomized controlled trials identified that evaluated pharmacologic therapies, including nonbenzodiazepine hypnotics (eszopiclone, zaleplon, zolpidem), an orexin receptor antagonist (suvorexant), melatonin agonists (prolonged-release melatonin, ramelteon), an antidepressant (doxepin), and a benzodiazepine hypnotic (temazepam). Most of these studies were of short duration and had a small sample size. Eszopiclone, zolpidem, and suvorexant had the strongest evidence of effectiveness in the general adult population for global and sleep outcomes. Doxepin had the strongest evidence of effectiveness for global and sleep outcomes among adults 55 years and older. Ramelteon did not improve global or sleep outcomes in a clinically meaningful way when compared with placebo. Very few benzodiazepine trials met eligibility criteria primarily because of short treatment duration, and data from the included studies were insufficient to assess sleep outcomes. Adverse events were mixed across studies, with few differences compared with placebo.
Psychological and behavioral interventions are considered first-line treatments for insomnia. Consistent with findings from this AHRQ study, guidelines from the American College of Physicians and the American Academy of Sleep Medicine recommend CBT as the first-line treatment for all adults with chronic insomnia disorder.6–8 When CBT for insomnia is not effective or does not achieve desired results, the American College of Physicians recommends using shared decision making when considering short-term medication therapy for chronic insomnia. The U.S. Food and Drug Administration has approved pharmacologic therapy for short-term use (four to five weeks) and suggests that medications not be used for extended periods. If insomnia does not improve after seven to 10 days of pharmacotherapy, the patient should be further evaluated.8
Editor's Note: American Family Physician SOR ratings are different from the AHRQ Strength of Evidence (SOE) ratings.
The associated AHRQ product was funded under Contract No. HHSA 290-2012-00016-I Task Order 12 from the Agency for Healthcare Research and Quality (AHRQ), U.S. Department of Health and Human Services (HHS). The authors of this manuscript are responsible for its content. Statements in the manuscript do not necessarily represent the official views of or imply endorsement by AHRQ or HHS.