Am Fam Physician. 2019;99(4):260-261
Author disclosure: No relevant financial affiliations.
Abaloparatide (Tymlos) is a daily injectable parathyroid hormone–related peptide analog. It is labeled for the treatment of postmenopausal osteoporosis in women at high risk of fracture because of a history of osteoporotic fracture, failure of treatment with other osteoporotic medications, smoking, excessive alcohol consumption, chronic steroid use, or a family history of fractures.1 Intermittent administration of abaloparatide has an anabolic effect on bone similar to teriparatide (Forteo), a recombinant human parathyroid hormone.1–3
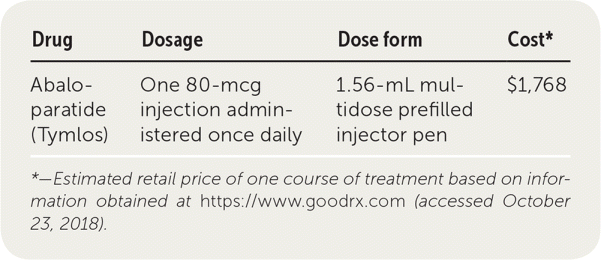
| Drug | Dosage | Dose form | Cost* |
|---|---|---|---|
| Abaloparatide (Tymlos) | One 80-mcg injection administered once daily | 1.56-mL multidose prefilled injector pen | $1,768 |
Safety
Abaloparatide is associated with a dose-dependent risk of osteosarcoma in rats. As such, its use is limited to a total duration of two years. It should not be used in patients with a history of Paget disease of bone or unexplained elevated alkaline phosphatase levels.1 Abaloparatide has been shown to increase serum calcium levels, and the manufacturer recommends against use in patients with preexisting hypercalcemia.1 The manufacturer also recommends measuring urinary calcium excretion in patients with active urolithiasis or a history of urolithiasis.1
Tolerability
Abaloparatide was well tolerated in clinical studies with few adverse effects that led to discontinuation. Orthostatic hypotension can occur up to four hours following injection in as many as 31% of patients, although the clinical significance of this occurrence is not known. The most common adverse effects of abaloparatide vs. placebo include palpitations (5% vs. 0.4%), nausea (8% vs. 3%), headache (8% vs. 6%), and dizziness (10% vs. 6%).1 Other adverse effects of abaloparatide include pain (9% vs. 7% with placebo), edema (10% vs. 3%), and injection site redness (58% vs. 28%).1
Effectiveness
Treatment with abaloparatide increases bone mineral density (BMD) over 24 months compared with placebo at the femoral neck (4.5%), the total hip (5.5%), and the lumbar spine (12.8%), regardless of baseline BMD.3 Abaloparatide will reduce the risk of new vertebral fractures, reduce the rate of nonvertebral fractures, delay the time to first incidence of nonvertebral fractures, and increase BMD in postmenopausal women with osteoporosis.3 These results are based on a study of 1,401 high-risk women that compared abaloparatide, teriparatide, and placebo in addition to daily supplemental calcium and vitamin D. Among participants, 24% had a previous vertebral fracture, and lumbar spine T-scores were –2.9 on average.1
After 18 months of treatment with abaloparatide, the rate of clinical fractures of any type or at any site was 3.3% compared with 5.9% with placebo (number needed to treat [NNT] = 37; 95% confidence interval [CI], 21 to 152).3 Treatment resulted in fewer vertebral fractures vs. placebo (absolute risk reduction = 3.6%; 95% CI, 2.1% to 5.4%); one additional patient will be prevented from experiencing a vertebral fracture for every 28 patients treated for 18 months (NNT = 28; 95% CI, 19 to 48).1 This rate was similar to that seen with teriparatide (absolute risk reduction = 3.4%). Abaloparatide has not been compared with other osteoporosis treatments such as bisphosphonates and denosumab (Prolia).4 It has not been evaluated for the prevention of hip fracture.
Price
A one-month supply of abaloparatide is approximately $1,768. In comparison, a 30-day supply of teriparatide costs about $3,359. Generic alendronate is considerably less expensive, costing about $10 for a one-month supply.
Simplicity
Abaloparatide is supplied in a refrigerated multidose pre-filled injector pen for subcutaneous injection once daily into the periumbilical region of the abdomen. Each prefilled pen delivers 30 doses, but the pen needles are not included and require a separate prescription. The site of injection should be rotated every day and abaloparatide should be administered at approximately the same time each day.1 The maximal treatment length of this medication is two years. There is no manufacturer recommendation for follow-up BMD testing once treatment is started.1
Bottom Line
Abaloparatide is a safe and effective medication used to reduce the likelihood of vertebral and nonvertebral fractures in women at high risk. However, it is expensive and requires daily injections. It has not been evaluated for its effect on decreasing hip fractures. Until a clear benefit is shown over other osteoporosis treatment options, abaloparatide should be reserved for select older women whose high risk of fractures may warrant its cost and difficulty of administration.