Am Fam Physician. 2019;99(9):577-583
Author disclosure: No relevant financial affiliations.
Key Clinical Issue
What are the benefits and harms of psychotherapies and pharmacologic agents for the treatment of adults with posttraumatic stress disorder (PTSD)?
Evidence-Based Answer
Cognitive behavior therapy (CBT) and CBT-mixed treatments had high strength of evidence for benefit in improving PTSD-related outcomes, such as reduced PTSD symptoms, reduced depression symptoms, and resolution of PTSD diagnosis. (Strength of Recommendation [SOR]: A, based on consistent, good-quality patient-oriented evidence.) Cognitive processing therapy, cognitive therapy, eye movement desensitization and reprocessing (EMDR), and narrative exposure therapy had moderate strength of evidence for benefit. (SOR: B, based on inconsistent or limited-quality patient-oriented evidence.) Fluoxetine, paroxetine, and venlafaxine had moderate strength of evidence for reducing PTSD symptoms. (SOR: B, based on inconsistent or limited-quality patient-oriented evidence.) There was insufficient evidence to compare psychotherapy with pharmacotherapy and to compare serious adverse events among treatments.1
Practice Pointers
Family physicians regularly diagnose and treat PTSD, which affects 8% of men and 20% of women in the general population.2 Similarly, 16% of female veterans receiving care in Veterans Health Administration facilities have PTSD.3 Male veterans have higher rates of PTSD than the general population; PTSD has been diagnosed in one out of four male combat veterans serving in Operation Enduring Freedom or Operation Iraqi Freedom.3 Untreated, PTSD can lead to substance abuse and suicide.2
This Agency for Healthcare Research and Quality (AHRQ) systematic review updates a 2013 report and includes 193 studies. Because several different outcome scales were used across trials (e.g., the Clinician-Administered PTSD Scale, which measures the presence and intensity of PTSD-related symptoms), the authors interpreted a standardized mean difference (SMD) of 0.5 (a medium effect size) as being clinically significant, although definitive thresholds for clinical significance have not been established.
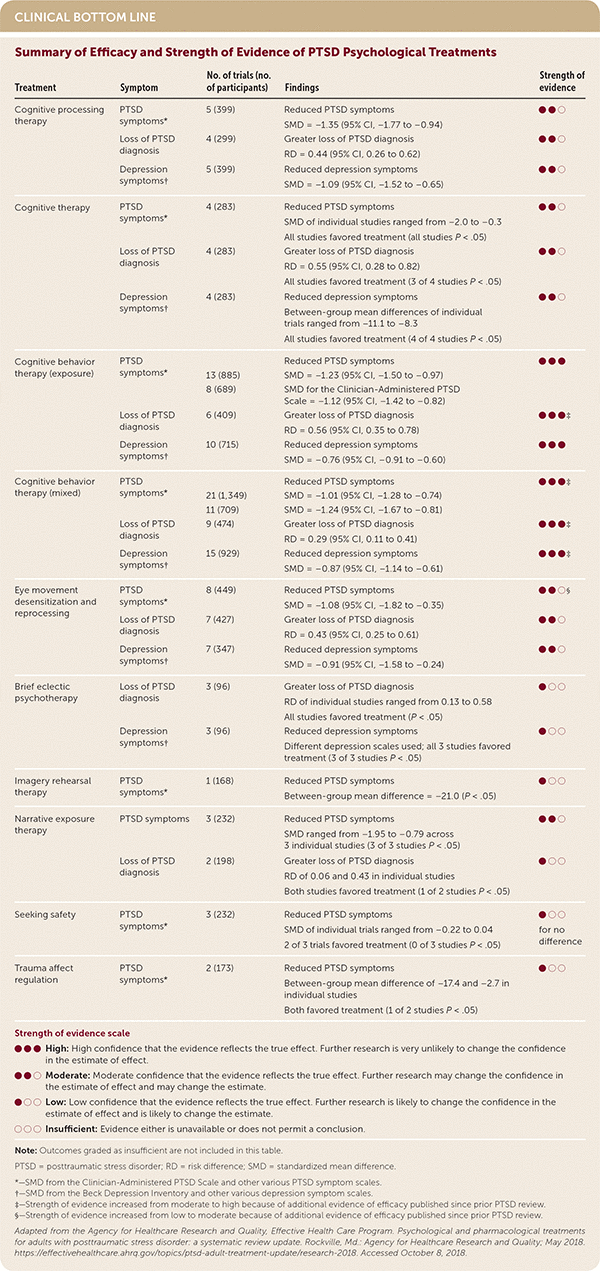
| Treatment | Symptom | No. of trials (no. of participants) | Findings | Strength of evidence |
|---|---|---|---|---|
| Cognitive processing therapy | PTSD symptoms* | 5 (399) | Reduced PTSD symptoms SMD = −1.35 (95% CI, −1.77 to −0.94) | ● ● ○ |
| Loss of PTSD diagnosis | 4 (299) | Greater loss of PTSD diagnosis RD = 0.44 (95% CI, 0.26 to 0.62) | ● ● ○ | |
| Depression symptoms† | 5 (399) | Reduced depression symptoms SMD = –1.09 (95% CI, –1.52 to –0.65) | ● ● ○ | |
| Cognitive therapy | PTSD symptoms* | 4 (283) | Reduced PTSD symptoms SMD of individual studies ranged from –2.0 to –0.3 All studies favored treatment (all studies P < .05) | ● ● ○ |
| Loss of PTSD diagnosis | 4 (283) | Greater loss of PTSD diagnosis RD = 0.55 (95% CI, 0.28 to 0.82) All studies favored treatment (3 of 4 studies P < .05) | ● ● ○ | |
| Depression symptoms† | 4 (283) | Reduced depression symptoms Between-group mean differences of individual trials ranged from –11.1 to –8.3 All studies favored treatment (4 of 4 studies P < .05) | ● ● ○ | |
| Cognitive behavior therapy (exposure) | PTSD symptoms* | Reduced PTSD symptoms | ● ● ● | |
| 13 (885) | SMD = –1.23 (95% CI, –1.50 to –0.97) | |||
| 8 (689) | SMD for the Clinician-Administered PTSD Scale = –1.12 (95% CI, –1.42 to –0.82) | |||
| Loss of PTSD diagnosis | 6 (409) | Greater loss of PTSD diagnosis RD = 0.56 (95% CI, 0.35 to 0.78) | ● ● ●‡ | |
| Depression symptoms† | 10 (715) | Reduced depression symptoms SMD = –0.76 (95% CI, –0.91 to –0.60) | ● ● ● | |
| Cognitive behavior therapy (mixed) | PTSD symptoms* | Reduced PTSD symptoms | ● ● ●‡ | |
| 21 (1,349) | SMD = –1.01 (95% CI, –1.28 to –0.74) | |||
| 11 (709) | SMD = –1.24 (95% CI, –1.67 to –0.81) | |||
| Loss of PTSD diagnosis | 9 (474) | Greater loss of PTSD diagnosis RD = 0.29 (95% CI, 0.11 to 0.41) | ● ● ●‡ | |
| Depression symptoms† | 15 (929) | Reduced depression symptoms SMD = –0.87 (95% CI, –1.14 to –0.61) | ● ● ●‡ | |
| Eye movement desensitization and reprocessing | PTSD symptoms* | 8 (449) | Reduced PTSD symptoms SMD = –1.08 (95% CI, –1.82 to –0.35) | ● ● ○§ |
| Loss of PTSD diagnosis | 7 (427) | Greater loss of PTSD diagnosis RD = 0.43 (95% CI, 0.25 to 0.61) | ● ● ○ | |
| Depression symptoms† | 7 (347) | Reduced depression symptoms SMD = –0.91 (95% CI, –1.58 to –0.24) | ● ● ○ | |
| Brief eclectic psychotherapy | Loss of PTSD diagnosis | 3 (96) | Greater loss of PTSD diagnosis RD of individual studies ranged from 0.13 to 0.58 All studies favored treatment (P < .05) | ● ○ ○ |
| Depression symptoms† | 3 (96) | Reduced depression symptoms Different depression scales used; all 3 studies favored treatment (3 of 3 studies P < .05) | ● ○ ○ | |
| Imagery rehearsal therapy | PTSD symptoms* | 1 (168) | Reduced PTSD symptoms Between-group mean difference = –21.0 (P < .05) | ● ○ ○ |
| Narrative exposure therapy | PTSD symptoms | 3 (232) | Reduced PTSD symptoms SMD ranged from –1.95 to –0.79 across 3 individual studies (3 of 3 studies P < .05) | ● ● ○ |
| Loss of PTSD diagnosis | 2 (198) | Greater loss of PTSD diagnosis RD of 0.06 and 0.43 in individual studies Both studies favored treatment (1 of 2 studies P < .05) | ● ○ ○ | |
| Seeking safety | PTSD symptoms* | 3 (232) | Reduced PTSD symptoms SMD of individual trials ranged from –0.22 to 0.04 2 of 3 trials favored treatment (0 of 3 studies P < .05) | ● ○ ○ for no difference |
| Trauma affect regulation | PTSD symptoms* | 2 (173) | Reduced PTSD symptoms Between-group mean difference of –17.4 and –2.7 in individual studies Both favored treatment (1 of 2 studies P < .05) | ● ○ ○ |
CBT focusing on imagined, written, virtual reality, and real-life exposures reduced PTSD symptoms by an SMD of –1.23 (95% CI, –1.5 to –0.97), led to a greater loss of PTSD diagnosis with a risk difference of 0.56 (95% CI, 0.35 to 0.78), and reduced depression symptoms by an SMD of –0.76 (95% CI, –0.91 to –0.60). CBT using mixed modalities (i.e., cognitive restructuring, exposure, guided imagery, mindfulness training, psychoeducation, self-monitoring, and/or stress management) reduced PTSD symptoms by an SMD of –1.01 (95% CI, –1.28 to –0.74), led to a greater loss of PTSD diagnosis with a risk difference of 0.29 (95% CI, 0.11 to 0.41), and reduced depression symptoms by an SMD of –0.87 (95% CI, –1.14 to –0.61). Both interventions were rated as having a high strength of evidence. Cognitive processing therapy, cognitive therapy, EMDR, and narrative exposure therapy had the same magnitude of PTSD outcomes reduction, with moderate strength of evidence because of imprecision in study results.
There was moderate strength of evidence to support the pharmacologic treatments fluoxetine (SMD = –0.28; 95% CI, –0.42 to –0.14; four studies, n = 835), paroxetine (SMD = –0.56 to –0.44; two studies, n = 348), and venlafaxine (SMD = –0.35 to –0.26; two studies, n = 687) for the reduction of PTSD symptoms. There was low strength of evidence that sertraline, prazosin, topiramate, olanzapine, and risperidone were effective for improving PTSD-related outcomes.
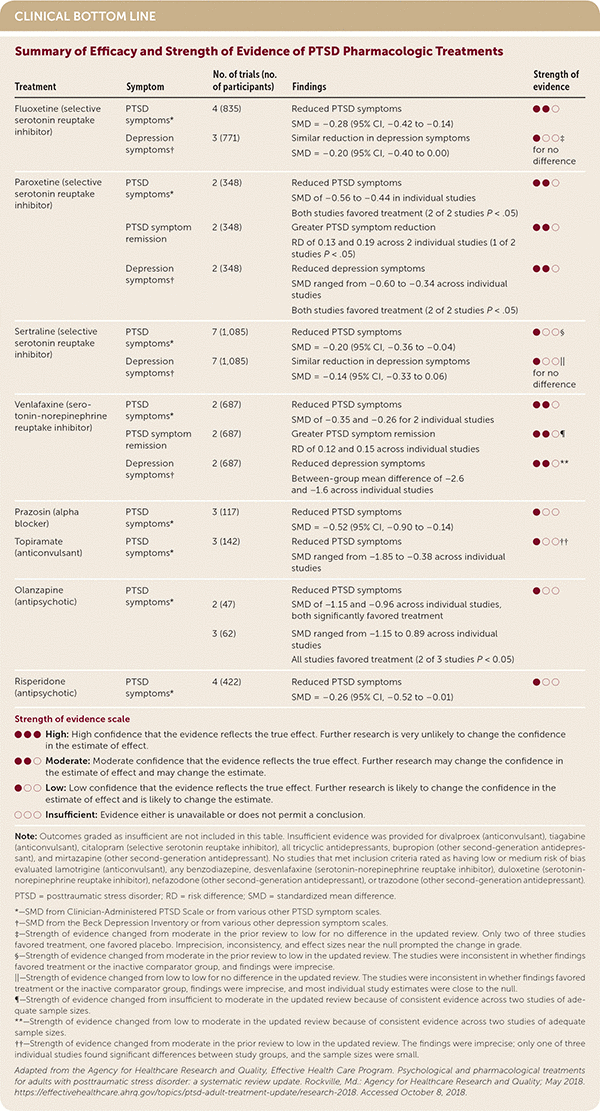
| Treatment | Symptom | No. of trials (no. of participants) | Findings | Strength of evidence |
|---|---|---|---|---|
| Fluoxetine (selective serotonin reuptake inhibitor) | PTSD symptoms* | 4 (835) | Reduced PTSD symptoms SMD = –0.28 (95% CI, –0.42 to –0.14) | ● ● ○ |
| Depression symptoms† | 3 (771) | Similar reduction in depression symptoms SMD = –0.20 (95% CI, –0.40 to 0.00) | ● ○ ○‡ for no difference | |
| Paroxetine (selective serotonin reuptake inhibitor) | PTSD symptoms* | 2 (348) | Reduced PTSD symptoms SMD of –0.56 to –0.44 in individual studies Both studies favored treatment (2 of 2 studies P < .05) | ● ● ○ |
| PTSD symptom remission | 2 (348) | Greater PTSD symptom reduction RD of 0.13 and 0.19 across 2 individual studies (1 of 2 studies P < .05) | ● ● ○ | |
| Depression symptoms† | 2 (348) | Reduced depression symptoms SMD ranged from –0.60 to –0.34 across individual studies Both studies favored treatment (2 of 2 studies P < .05) | ● ● ○ | |
| Sertraline (selective serotonin reuptake inhibitor) | PTSD symptoms* | 7 (1,085) | Reduced PTSD symptoms SMD = –0.20 (95% CI, –0.36 to –0.04) | ● ○ ○§ |
| Depression symptoms† | 7 (1,085) | Similar reduction in depression symptoms SMD = –0.14 (95% CI, –0.33 to 0.06) | ● ○ ○|| for no difference | |
| Venlafaxine (serotonin-norepinephrine reuptake inhibitor) | PTSD symptoms* | 2 (687) | Reduced PTSD symptoms SMD of –0.35 and –0.26 for 2 individual studies | ● ● ○ |
| PTSD symptom remission | 2 (687) | Greater PTSD symptom remission RD of 0.12 and 0.15 across individual studies | ● ● ○¶ | |
| Depression symptoms† | 2 (687) | Reduced depression symptoms Between-group mean difference of –2.6 and –1.6 across individual studies | ● ● ○** | |
| Prazosin (alpha blocker) | PTSD symptoms* | 3 (117) | Reduced PTSD symptoms SMD = –0.52 (95% CI, –0.90 to –0.14) | ● ○ ○ |
| Topiramate (anticonvulsant) | PTSD symptoms* | 3 (142) | Reduced PTSD symptoms SMD ranged from –1.85 to –0.38 across individual studies | ● ○ ○†† |
| Olanzapine (antipsychotic) | PTSD symptoms* | 2 (47) | Reduced PTSD symptoms SMD of –1.15 and –0.96 across individual studies, both significantly favored treatment | ● ○ ○ |
| 3 (62) | SMD ranged from –1.15 to 0.89 across individual studies All studies favored treatment (2 of 3 studies P < 0.05) | |||
| Risperidone (antipsychotic) | PTSD symptoms* | 4 (422) | Reduced PTSD symptoms SMD = –0.26 (95% CI, –0.52 to –0.01) | ● ○ ○ |
The AHRQ review findings are similar to the recommendations from the U.S. Department of Veterans Affairs (VA)/U.S. Department of Defense (DoD) clinical practice guideline.3 This guideline states that the trauma-focused psychotherapies with the strongest evidence are prolonged exposure, cognitive processing therapy, and EMDR; there is sufficient evidence to also recommend narrative exposure and other types of CBT. Non–trauma-focused psychotherapies that were reported as potentially helpful include stress inoculation training, present-centered therapy, and interpersonal psychotherapy. The pharmacotherapies recommended in the VA/DoD guideline include fluoxetine, paroxetine, venlafaxine, and sertraline; the guideline recommends against the use of atypical antipsychotic medications, topiramate, and amitriptyline.
For the family physician in the clinic, the VA/DoD guideline recommends screening adults at risk of PTSD (e.g., those exposed to traumatic events such as war, natural disaster, or violence) with the Primary Care PTSD Screen (available at https://www.ptsd.va.gov/professional/assessment/screens/pc-ptsd.asp) or the PTSD Checklist (available at https://www.mirecc.va.gov/cih-visn2/Documents/Clinical/PCL-5_with_Info_Sheet.pdf). Those who screen positive should have a structured interview using a tool such as the Clinician-Administered PTSD Scale. Once the diagnosis is made, the VA/DoD guideline recommends starting with individual trauma-focused psychotherapy, based on evidence that psychotherapy results in a greater change in symptoms with more persistent benefits. Pharmacotherapy may be used when psychotherapy is not available.3
Editor’s Note: AFP’s SOR ratings are different from the AHRQ Strength of Evidence (SOE) ratings. Dr. Saguil is a contributing editor for AFP.
The views expressed in this piece are the author’s own and do not reflect the views of the Uniformed Services University of the Health Sciences, the U.S. Army, or the Department of Defense.