Am Fam Physician. 2019;99(12):781-782
Author disclosure: No relevant financial affiliations.
| Drug | Dosage | Dose form | Cost* |
|---|---|---|---|
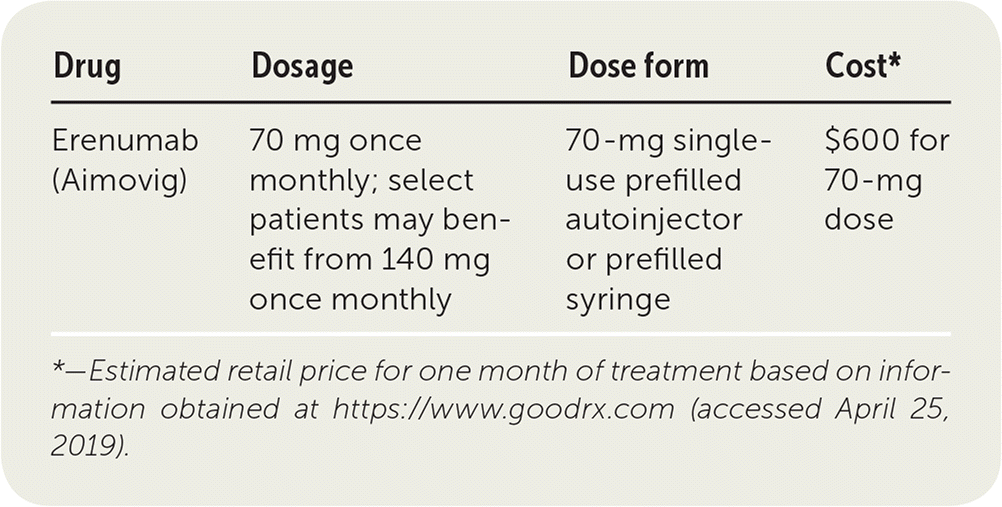
| Erenumab (Aimovig) | 70 mg once monthly; select patients may benefit from 140 mg once monthly | 70-mg single-use prefilled autoinjector or prefilled syringe | $600 for 70-mg dose |
Safety
Erenumab, which has been studied in 2,537 patients with migraine who received at least one dose, has no notable drug-drug interactions or any contraindications. There is no clinically significant increase in blood pressure when sumatriptan (Imitrex) is added for breakthrough migraine pain. Erenumab is available as a prefilled autoinjector that contains a latex derivative and may cause a reaction in patients with latex allergy.1 Erenumab should not be used in patients younger than 18 years because safety and effectiveness have not been established. No increase in birth defects or miscarriage has been observed, and there are no data on presence in breast milk or effect on milk production.1
Tolerability
Injection site reactions (pain, erythema, and pruritus) are the most common adverse effects, experienced by 5% to 6% of patients. Allowing erenumab to come to room temperature for 30 minutes before administration may decrease injection site reactions. Patients receiving 140-mg doses also reported experiencing constipation (3%). In a series of 2,199 patients taking erenumab for three to six months, 1.3% discontinued therapy because of adverse effects.3–5
Effectiveness
Erenumab has been evaluated for prophylaxis in patients with episodic or chronic migraine. In patients with episodic migraine (i.e., an average of eight migraine days per month), after four to six months of treatment, 43.3% of patients receiving 70 mg and 50.0% of patients receiving 140 mg will have at least a 50% reduction in mean monthly migraine days vs. 26.6% of those receiving placebo (numbers needed to treat = 6 and 4, respectively). Erenumab will decrease the number of migraine days by an additional 1.4 days per month at 70 mg and 1.9 days per month at 140 mg when compared with placebo.3
In patients with chronic migraine (i.e., an average of 18 migraine days per month), 39.9% of patients receiving 70 mg of erenumab and 41.2% of patients receiving 140 mg will have at least a 50% reduction in mean monthly migraine days vs. 23.5% of patients receiving placebo after three months of treatment (number needed to treat = 6 for both comparisons). Erenumab will decrease the number of migraine days by 2.4 per month with either dose.4
Although not formally studied, a decrease in the number of migraine days may improve quality of life, decrease the number of days lost from work, and reduce the use of medication for acute migraine pain. Erenumab has not been compared directly with other prophylactic therapies such as metoprolol (Lopressor), propranolol (Inderal), divalproex sodium (Depakote), or topiramate (Topamax). Existing literature suggests these treatments reduce migraine days by one to two and a half days per month.6
Price
Erenumab costs about $600 for 70 mg per month and $1,200 for 140 mg per month, making it similar in price to other calcitonin gene–related peptide antagonists on the market.7 Compared with oral migraine prophylaxis medications, which are available as generic formulations, it is significantly more expensive.
Simplicity
Erenumab is administered once monthly in dosages of 70 mg or 140 mg. It is available in 70-mg single-use pens for self-administered subcutaneous injection. Patients receiving 140 mg should be instructed to administer two injections together. Erenumab must be kept refrigerated but can be stored at room temperature for up to seven days. There are no dose adjustments needed for renal or hepatic impairment. Erenumab has not been studied in patients with renal dysfunction (i.e., creatinine clearance less than 30 mL per minute per 1.73 m2 [0.50 mL per second per m2]) and should be avoided in these patients.1 Studies do not indicate which patient populations are more likely to benefit from the higher dose.
Bottom Line
Erenumab is a safe option for migraine prophylaxis in adults. However, it is expensive and should be reserved for patients who have experienced intolerable adverse effects while taking oral migraine prophylaxis medication or who have poor adherence to daily preventive treatment. It is not recommended for use in patients younger than 18 years. Most patients should receive 70 mg once monthly because there is no clear dose-response relationship.