
Am Fam Physician. 2019;100(3):online
Author disclosure: No relevant financial affiliations.

Details for This Review
Study Population: Asymptomatic adults 45 to 80 years of age
Efficacy End Points: Death from colorectal cancer, death from any cause
Harm End Points: Bleeding, perforation, or death resulting from follow-up colonoscopy or surgery
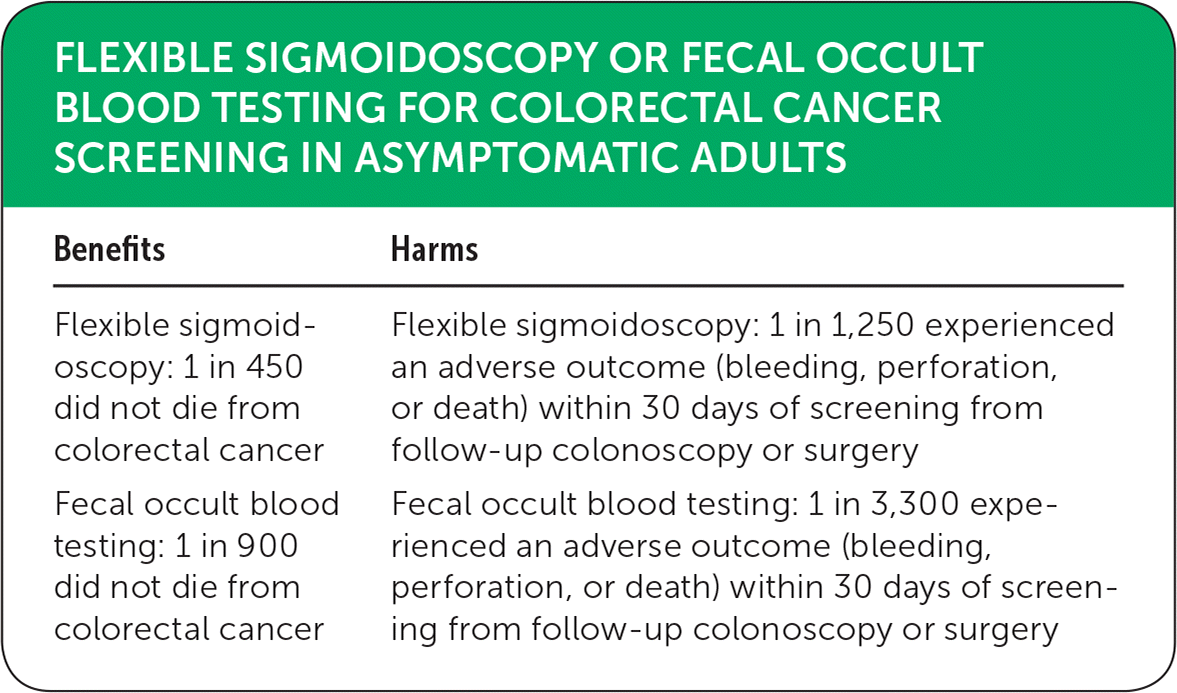
| Benefits | Harms |
|---|---|
| Flexible sigmoidoscopy: 1 in 450 did not die from colorectal cancer | Flexible sigmoidoscopy: 1 in 1,250 experienced an adverse outcome (bleeding, perforation, or death) within 30 days of screening from follow-up colonoscopy or surgery |
| Fecal occult blood testing: 1 in 900 did not die from colorectal cancer | Fecal occult blood testing: 1 in 3,300 experienced an adverse outcome (bleeding, perforation, or death) within 30 days of screening from follow-up colonoscopy or surgery |
Narrative: Colorectal cancer continues to be the third leading cause of cancer death in the United States.1,2 The aim of screening is to reduce mortality through early detection.2,3 Several methods of colorectal cancer screening are available, including stool-based testing (guaiac fecal occult blood test, fecal immunochemical test), endoscopic methods (sigmoidoscopy, colonoscopy), and imaging methods with computed tomographic colonography among other new techniques.4 Fecal occult blood testing (FOBT) and flexible sigmoidoscopy have not been compared directly to determine whether either is the superior screening modality.1
The Cochrane meta-analysis cited here assessed the effectiveness of FOBT and flexible sigmoidoscopy as colorectal cancer screening modalities in asymptomatic patients.1 The primary outcome measured was colorectal cancer mortality. The meta-analysis included nine randomized controlled trials (RCTs), with 338,467 participants in the screening group and 405,919 in the control group. Flexible sigmoidoscopy (n = 165,773) as a screening modality was compared with no screening (n = 414,744) in five RCTs; guaiac-based FOBT (n = 172,734) was compared with no screening (n = 329,642) in four RCTs. Colorectal cancer mortality was lower with flexible sigmoidoscopy (relative risk [RR] = 0.72; 95% CI, 0.65 to 0.79; absolute risk difference [ARD] = 0.22%; number needed to treat [NNT] = 450) and FOBT (RR = 0.86; 95% CI, 0.80 to 0.92; ARD = 0.11%; NNT = 900) when compared with no screening. In other words, 450 patients would need to be screened with flexible sigmoidoscopy to prevent one death resulting from colorectal cancer; whereas, 900 patients would need to be screened with FOBT to prevent one colorectal cancer death. The latter can be explained with increased follow-up colonoscopies for positive FOBT results. Despite this, neither of these modalities reduce all-cause mortality (seven trials). Major complications (bleeding, perforation, or death) within 30 days of screening, follow-up colonoscopy or surgery occurred in 0.03% and 0.08% of participants in the FOBT and flexible sigmoidoscopy trials, respectively.1
Caveats: Despite evidence of flexible sigmoidoscopy and FOBT reducing colorectal cancer mortality, there are caveats to consider.1 The meta-analysis does not provide a clear answer regarding a superior screening modality.1 In the absence of direct evidence, it cannot be concluded whether the net benefit or harm of one modality is greater than the other.1 The decision to choose one test over another should be a shared decision made by the patient and the physician. In a patient with no family history of colon cancer, FOBT could be the first logical choice because it is less invasive. The U.S. Preventive Services Task Force guidelines recommend colon cancer screening for adults between 50 and 75 years of age.5
This meta-analysis provides high-quality evidence that flexible sigmoidoscopy and FOBT both reduce the risk of death from colorectal cancer.1 Therefore, we assign a color recommendation of green (benefits greater than harms) to both screening tests.
Copyright © 2019 MD Aware, LLC (theNNT.com). Used with permission.
This series is coordinated by Dean A. Seehusen, MD, MPH, AFP Assistant Medical Editor, and Daniel Runde, MD, from the NNT Group.
A collection of Medicine by the Numbers published in AFP is available at https://www.aafp.org/afp/mbtn.
