
Am Fam Physician. 2019;100(4):227-235
Related letter: Electronic Cigarettes: More Questions Than Answers
Patient information: See related handout on e-cigarettes, vaping, and Juuls, written by the authors of this article.
Electronic cigarettes (e-cigarettes) are popular devices designed to heat a liquid solution, often containing nicotine, that generates an inhaled aerosol, or vapor. e-Cigarettes have been marketed as healthier alternatives to traditional cigarettes. Thus, most adult users are current or former smokers who use e-cigarettes to reduce or quit cigarette smoking. Switching completely from cigarettes to e-cigarettes is associated with reduced toxicant exposure and reduced short-term respiratory symptoms; however, long-term health effects of e-cigarettes are unknown. Although a recent randomized trial suggests that e-cigarettes may promote smoking cessation, systematic reviews have had low certainty of evidence regarding cessation. e-Cigarettes pose several potential health risks, including exposure to heavy metals and toxicants, and nicotine poisoning. e-Cigarettes are also popular among youth, with rates of e-cigarette use surpassing those of cigarette use in this population. Youth e-cigarette use is associated with increased risk of subsequent cigarette and marijuana use. Screening for e-cigarette use in youth and adults, including pregnant women, in conjunction with screening for tobacco use, is advised. Education and interventions to prevent e-cigarette use should be provided to all youth. Youth should be counseled to stop using nicotine/tobacco products, including e-cigarettes. Although the impact of e-cigarette use in pregnancy is unknown, nicotine is a teratogen; thus, pregnant women should be counseled to abstain from using all nicotine/tobacco products.
What Is an e-Cigarette?
Electronic cigarettes (e-cigarettes) are battery-operated devices that heat a liquid solution to generate an aerosol that users inhale. The liquid solution in most e-cigarettes contains nicotine, the primary addictive agent in traditional cigarettes, and is available in a variety of flavors. Because e-cigarette aerosol is commonly called vapor, patients may refer to e-cigarette use as vaping. Currently, the most popular e-cigarette brand is Juul, which uses small, rectangular flavored cartridges that resemble a thumb drive. Most adults report using e-cigarettes to reduce or stop cigarette smoking.
WHAT IS NEW ON THIS TOPIC
Among U.S. youth, rates of e-cigarette use surpassed rates of traditional cigarette use in 2014. In 2017, one in five U.S. high school students reported using e-cigarettes in the previous year.
A recent meta-analysis indicated that among teens and young adults who have never smoked, the odds of smoking initiation are three to six times greater in those who have ever used e-cigarettes than in those who have never used e-cigarettes.
The U.S. Preventive Services Task Force concludes that the current evidence is insufficient to recommend e-cigarettes for tobacco cessation in adults, including pregnant women.
e-cigarette = electronic cigarette.
EVIDENCE SUMMARY
Since the introduction of e-cigarettes in the United States in 2006, use of and interest in these devices have increased dramatically.1 e-Cigarettes were initially marketed as safer alternatives to conventional cigarettes because they do not combust tobacco, which is linked to many of the adverse health effects of cigarette smoking.2,3 e-Cigarettes generate an aerosol, or vapor, by heating a liquid typically composed of nicotine, a humectant (propylene glycol or glycerin), and flavorings.3 Figure 1 shows different types of e-cigarettes.4

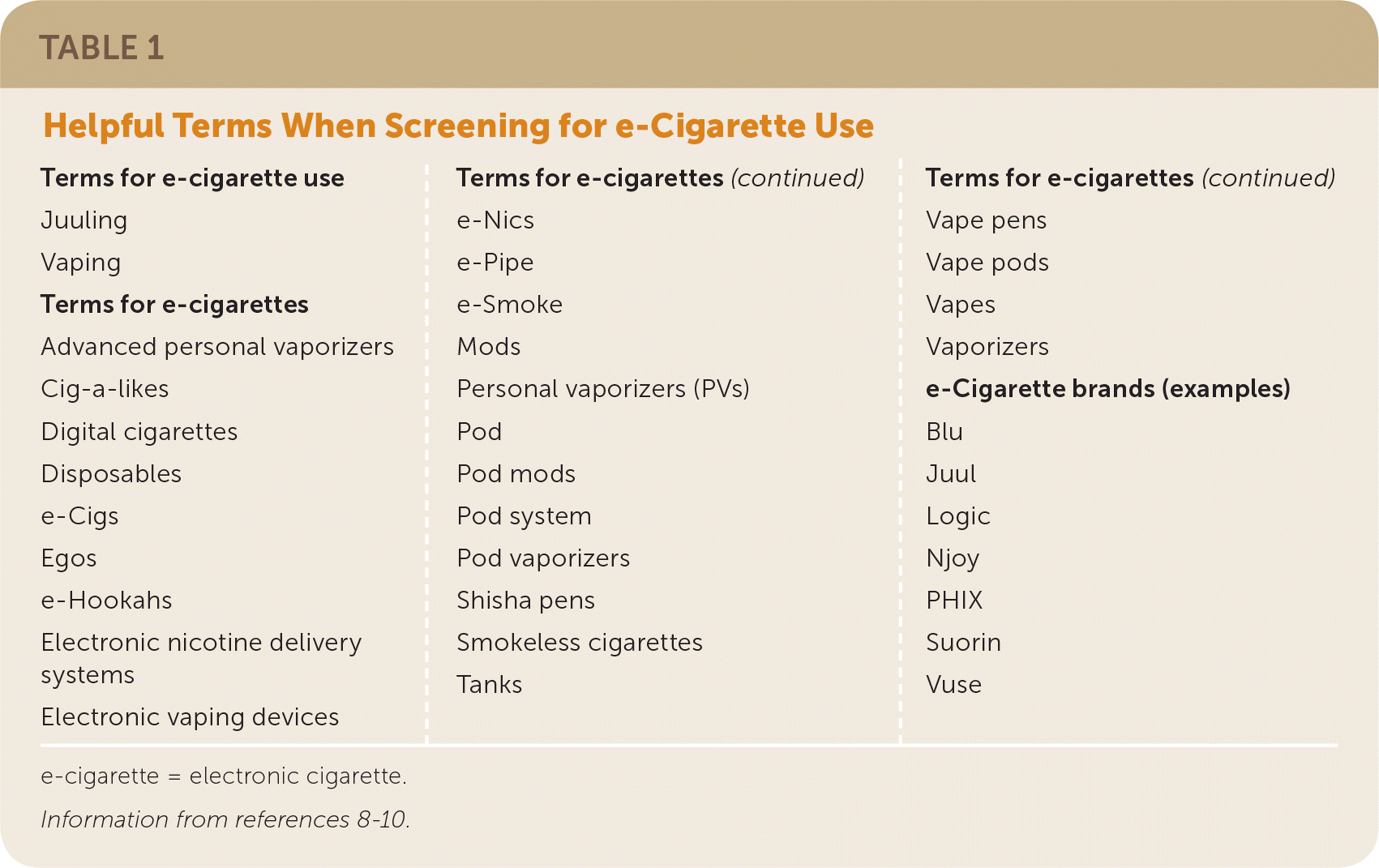
Although 99% of e-cigarettes contain nicotine, teens are more likely to report using e-cigarettes for substances other than nicotine, including flavors and marijuana.5,6 e-Cigarettes can be purchased in retail stores and online and come in more than 7,000 flavors, which may increase their appeal among youth and nonsmokers.7 Many users, especially teens and young adults, refer to e-cigarettes by brand name or a slang term (Table 1).8–10
| Terms for e-cigarette use |
| Juuling |
| Vaping |
| Terms for e-cigarettes |
| Advanced personal vaporizers |
| Cig-a-likes |
| Digital cigarettes |
| Disposables |
| e-Cigs |
| Egos |
| e-Hookahs |
| Electronic nicotine delivery systems |
| Electronic vaping devices |
| e-Nics |
| e-Pipe |
| e-Smoke |
| Mods |
| Personal vaporizers (PVs) |
| Pod |
| Pod mods |
| Pod system |
| Pod vaporizers |
| Shisha pens |
| Smokeless cigarettes |
| Tanks |
| Vape pens |
| Vape pods |
| Vapes |
| Vaporizers |
| e-Cigarette brands (examples) |
| Blu |
| Juul |
| Logic |
| Njoy |
| PHIX |
| Suorin |
| Vuse |
In 2016 in the United States, 15% of adults reported any lifetime e-cigarette use, and 3% reported use in the previous 30 days.11 Most adult users are current or former smokers who use e-cigarettes to reduce or quit traditional cigarette smoking.11 Although daily use of e-cigarettes is associated with a greater likelihood of smoking cessation, most adults and youth do not use e-cigarettes daily.12–14 Among U.S. youth, rates of e-cigarette use surpassed rates of traditional cigarette use in 2014.15 In 2017, one in five U.S. high school students reported using e-cigarettes in the previous year.16
The e-cigarette brand Juul is part of the newest generation of e-cigarettes called pod mods, which are rechargeable with replaceable cartridges.17 Juul's popularity may stem from its sleek, discreet, and easy-to-use design, as well as its more palatable nicotine solution and appealing flavor options.17 Youth Juul users do not report increased e-cigarette use compared with users of other e-cigarette brands, but Juul users are more likely to have tried to quit e-cigarettes, which is suggestive of nicotine dependence.18
What Are the Purported Health Benefits of e-Cigarettes for Smokers Attempting Cessation?
Although long-term health outcomes from e-cigarettes are unknown, there is evidence that switching completely from cigarettes to e-cigarettes reduces exposure to non-nicotine toxic chemicals and carcinogens. Some evidence from small-scale studies suggests that switching from cigarettes to e-cigarettes improves short-term health outcomes.
EVIDENCE SUMMARY
The impact of continued e-cigarette use on long-term health outcomes (e.g., cardiovascular disease, cancer) is unknown. However, there is evidence that e-cigarette use is associated with decreased toxicity and fewer short-term health risks vs. traditional cigarettes.3 Switching completely from cigarettes to e-cigarettes is associated with reduced exposure to non-nicotine toxic chemicals and carcinogens.3 In one study, smokers who switched completely to e-cigarettes had a 64% reduction in a tobacco-specific biomarker after two weeks.19
Switching from cigarettes to e-cigarettes has also been associated with improved short-term respiratory outcomes, although most studies had small sample sizes.3 In one study, healthy smokers were randomized to switch to e-cigarettes with varying nicotine levels or to not switch to e-cigarettes. At the 52-week follow-up, self-reported cough/phlegm symptoms were reduced and researcher-measured forced expiratory flow was increased in cigarette smokers who switched to e-cigarettes.20 In a retrospective chart review of 48 patients with chronic obstructive pulmonary disease (COPD), including 24 daily e-cigarette users and 24 matched traditional cigarette smokers, e-cigarette users showed decreased COPD exacerbations, improved patient-reported COPD health status, and a reversal in expiratory volume decline over 24 months of follow-up.21
What Are the Potential Harms of e-Cigarettes?
Most e-cigarettes contain nicotine, which is highly addictive. e-Cigarette aerosol also delivers toxicants, including heavy metals, although in lower concentrations than cigarette smoke. Little is known about the health impact of inhaling humectants and flavorings from e-cigarettes. Harm has occurred from contact with the liquid nicotine solution, especially in children, and exploding batteries. It is critical to counsel all parents and adults who spend time around children about these risks and the importance of safe storage practices.
There are no data regarding the risks of e-cigarette use, including the relative risks vs. cigarette use, in pregnant women. However, nicotine itself is teratogenic, even in the absence of combustion byproducts. Family physicians should encourage and assist pregnant women with complete cessation of all nicotine/tobacco products during pregnancy.
Nicotine may have a detrimental impact on brain development in youth, and e-cigarettes may act as a gateway drug for cigarette or marijuana use. Youth are also susceptible to nicotine addiction. Flavored products, such as Juul, may be especially appealing to youth. Family physicians should counsel youth to stop all nicotine/tobacco products, including e-cigarettes.
EVIDENCE SUMMARY
Nicotine is the primary addictive component in tobacco products; thus, there is also a risk of addiction with e-cigarettes. Nicotine concentrations listed on e-cigarette labels are often inaccurate.22 Liquids and vapor from e-cigarettes have also been shown to contain toxicants, including heavy metals (e.g., chromium, lead, arsenic), volatile organic compounds, and tobacco-specific nitrosamines, although in lower concentrations than cigarettes.23 Exposure to nicotine and toxicants in e-cigarettes is highly variable, depending on the device and user behavior, making it difficult to determine risk.3 Although humectants, which make up the majority of e-liquid volume, and many e-cigarette flavorings are generally recognized by the U.S. Food and Drug Administration (FDA) as safe for ingestion, little testing has been conducted on the safety of inhalation in humans.3
The liquid solution in e-cigarettes contains high concentrations of nicotine that can cause nicotine poisoning through ingestion, dermal contact, or inhalation.24 e-Cigarette liquid ingestion is more hazardous than cigarette ingestion. Children exposed to e-cigarette liquid have more than five times the risk of hospital admission than children exposed to cigarettes.24 Small children are especially vulnerable to medical problems from e-cigarette exposure.24 Symptoms of nicotine poisoning include dizziness, tachycardia, vomiting, and seizures. Defective e-cigarette batteries may also explode, causing burns.25
e-Cigarettes are perceived as less harmful than cigarettes during pregnancy and as useful products for smoking cessation in pregnant women.28 Cigarette use during pregnancy is associated with low birth weight, preterm birth, and sudden infant death syndrome.29 The risks of e-cigarette use, including relative risks vs. cigarette use and risks of dual use (cigarettes and e-cigarettes used concurrently), for pregnant women and fetal development are unknown.3 However, although tobacco combustion byproducts are considered more harmful than nicotine for nonpregnant adults, nicotine itself is a developmental toxicant when used during pregnancy and is characterized by the FDA as teratogenic to human fetuses.29 In animal models, exposure to prenatal nicotine upregulates nicotine receptors, leading to changes in offspring brain structure and function.29
Tobacco use is often initiated in adolescence, a key period for brain development. The effects of e-cigarettes on human brain development are currently unknown. In animal models, nicotine lowers impulse control circuits, alters circuits related to mood and addiction, and affects brain regions dedicated to attention and learning.4 There is growing evidence that e-cigarette use increases risk of cigarette initiation.30 A recent meta-analysis indicated that in teens and young adults who have never smoked, the odds of smoking initiation are three to six times greater in those who have ever used e-cigarettes than in those who have never used e-cigarettes.31 One study also showed links between e-cigarette use and subsequent marijuana use in teens.32 e-Cigarettes may also be used to vaporize marijuana or cannabis oil. Sweet flavors, such as “mango fruit medley” and “candy,” may be particularly appealing to youth, who can become addicted to nicotine and may have difficulty quitting e-cigarettes.33,34 Rates of secondhand exposure to e-cigarette vapor among youth are also high at 18% for middle schoolers and 29% for high schoolers.35 Secondhand e-cigarette exposure increases the risk of an asthma attack by 27% in youth with asthma.36
Can e-Cigarettes Help with Smoking Cessation?
e-Cigarettes are not FDA-approved smoking cessation devices; however, recent evidence indicates that they may improve cessation outcomes compared with nicotine replacement therapy. It is difficult to give specific dosing advice, because actual nicotine doses can vary from what is indicated on package labels and doses can depend on the device and user behavior. Also, there is a possibility of continued e-cigarette use, with or without concurrent cigarette use, following a trial.
EVIDENCE SUMMARY
Research on this topic is evolving, and there is low certainty of evidence.12,37 Evidence from a limited number of randomized controlled trials and a Cochrane review suggests that e-cigarettes containing nicotine may help with smoking cessation or reduction.12,37 However, some trials have found no impact of e-cigarettes on smoking cessation beyond usual care.38 Unlike nicotine replacement therapy, the advertised nicotine dose on the labeling of e-cigarettes is not always consistent with laboratory analysis of the e-cigarette liquid, and the device and user behavior may affect the dose of nicotine received.3 There are also rapid and ongoing changes in e-cigarette technology that may improve the impact on smoking cessation (e.g., by delivering nicotine more quickly and in higher doses to better approximate nicotine delivery by cigarettes). For example, a recent randomized controlled trial of refillable e-cigarettes (vs. nonrefillable e-cigarettes used in prior trials) and ongoing behavioral counseling found a statistically significant difference in one-year abstinence rates between smokers using the e-cigarettes (18%) and those using nicotine replacement therapy (10%).39 However, 80% of participants randomized in the e-cigarette group were still using e-cigarettes after 52 weeks, whereas only 9% of the nicotine replacement therapy group were still using the therapy.39
Clinical Recommendations
E-CIGARETTES AND SMOKING CESSATION
The following summarizes recommendations for counseling patients on the role of e-cigarettes in smoking cessation.
Ask youth and adults, including pregnant women, about e-cigarette use when screening for tobacco use.40,41 Multiple terms for e-cigarettes should be used during screening to help identify all e-cigarette users (Table 1).8–10
Explain that although e-cigarettes are likely less harmful than cigarettes,3 they are not FDA-approved cessation devices. Because e-cigarettes are new, their safety is largely unknown, especially long-term health outcomes. Patients should first be counseled to quit using evidence-based smoking cessation guidelines as supported by U.S. Preventive Services Task Force (USPSTF) recommendations, including behavioral counseling and/or FDA-approved smoking cessation products.40 If evidence-based methods are ineffective or the patient does not comply with them, e-cigarettes may be discussed. However, the USPSTF finds that there is insufficient evidence to recommend e-cigarettes for smoking cessation.40
Recommend that patients switch completely to e-cigarettes (noting that there is no evidence of health benefits from dual use of e-cigarettes and cigarettes) and avoid flavored products.3
Offer ongoing behavioral support. Following complete smoking cessation, patients should set a quit date for stopping e-cigarette use. Current e-cigarette and cigarette use should be assessed at each appointment and adverse effects monitored.41
Counsel patients to avoid exposing others to secondhand e-cigarette vapor and about the potential dangers of the devices and liquids.24 Help patients with children develop a plan to safely store their devices and liquids.
E-CIGARETTES AND TEENS
The following summarizes counseling recommendations for teens regarding e-cigarette use.
Screening for all nicotine and tobacco products, especially e-cigarettes, is critical for youth.41 Provide teens with brief education in the context of tobacco screening and prevention, as recommended by the USPSTF.41
Youth should be counseled to quit all nicotine products, including e-cigarettes, using evidence-based strategies. Explain that nicotine has a greater effect on their brains, which will continue to develop until their 20s, including impacting brain pathways that control attention, mood, learning, and addiction. 34 Alert them that nicotine levels provided on e-cigarette labels may not be accurate. It may be helpful to share information about the financial links between cigarette and e-cigarette manufacturers (e.g., Juul is now owned by Altria, which makes cigarettes) to reduce the novelty of e-cigarettes.
ADDITIONAL INFORMATION

| What are the health effects of e-cigarettes? |
| e-Cigarettes typically contain nicotine, which is highly addictive.3 |
| e-Cigarette aerosol, or vapor, contains harmful metals and chemicals but in lower concentrations than conventional cigarette smoke.1,3 |
| In adults, switching completely from traditional cigarettes to e-cigarettes is associated with reduced exposure to non-nicotine toxic chemicals and carcinogens.3 |
| The long-term health effects of e-cigarettes are unknown.3 |
| e-Cigarette liquids can cause nicotine poisoning through ingestion, inhalation, or dermal contact, and ingestion can be fatal.24 |
| Can e-cigarettes help with smoking cessation? |
| e-Cigarettes may be helpful for smoking cessation if U.S. Food and Drug Administration–approved cessation devices are ineffective.12,37,38 |
| Many adults who use e-cigarettes do not quit smoking and instead continue to use both e-cigarettes and conventional cigarettes (dual use).3,12,37 |
| What is known about e-cigarette use and pregnancy? |
| Risks of e-cigarettes, including relative risks compared with cigarettes, for maternal and fetal health are unknown.3 |
| Nicotine is a teratogen, independent of tobacco combustion.29 |
| How should e-cigarette use be approached with youth? |
| e-Cigarette use in youth may lead to transition to combustible tobacco products.31 |
| Teens can become addicted to nicotine and may have difficulty quitting e-cigarettes.34 |
| Secondhand exposure to e-cigarettes may increase the risk of an asthma attack in youth with asthma.36 |
Data Sources: A PubMed search was completed in Clinical Queries using the key terms e-cigarette and electronic cigarette. The search included systematic reviews, meta-analyses, and reviews of clinical trials. We also reviewed the Cochrane database; the National Academies of Sciences, Engineering, and Medicine Public Health Consequences of e-Cigarettes report,3 which is a comprehensive and systematic assessment and review of the literature; and the surgeon general's report on e-cigarette use among youth and young adults.4 Search dates: July and December 2018, and March 2019.
This work was supported in part by grants from the National Institutes of Health (NIH): 1R01DA045492 01A1 and T32AA007459. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The NIH had no role in writing the manuscript or the decision to submit the paper for publication.
The authors thank Thomas Eissenberg, PhD, for his input on this manuscript and Pamela Borek for administrative assistance.
