
Am Fam Physician. 2019;100(4):236-238
As published by the USPSTF.
Summary of Recommendation and Evidence
The USPSTF recommends prophylactic ocular topical medication for all newborns to prevent gonococcal ophthalmia neonatorum (Table 1). A recommendation.
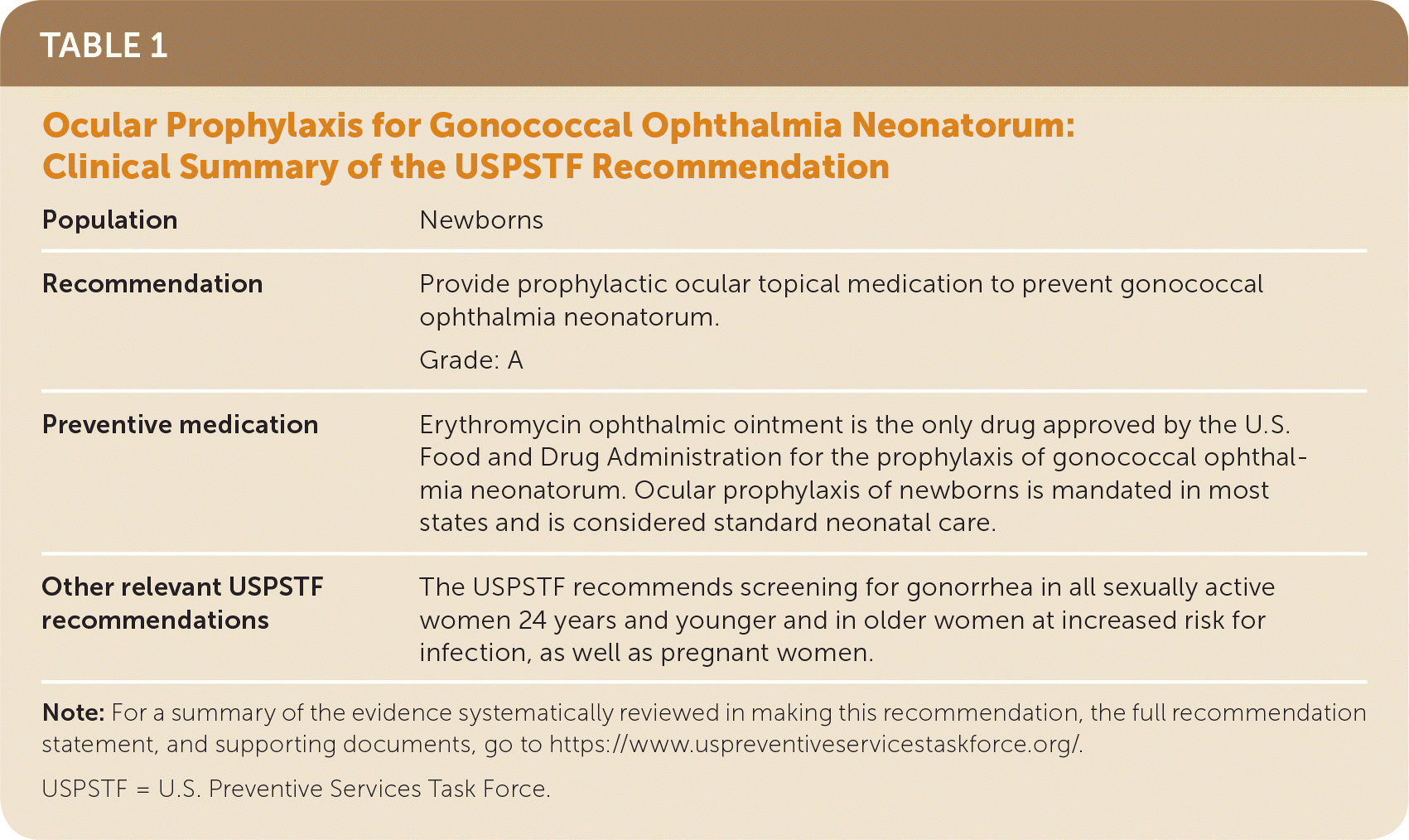
| Population | Newborns |
| Recommendation | Provide prophylactic ocular topical medication to prevent gonococcal ophthalmia neonatorum. Grade: A |
| Preventive medication | Erythromycin ophthalmic ointment is the only drug approved by the U.S. Food and Drug Administration for the prophylaxis of gonococcal ophthalmia neonatorum. Ocular prophylaxis of newborns is mandated in most states and is considered standard neonatal care. |
| Other relevant USPSTF recommendations | The USPSTF recommends screening for gonorrhea in all sexually active women 24 years and younger and in older women at increased risk for infection, as well as pregnant women. |
Rationale
IMPORTANCE
In the United States, the rate of gonococcal ophthalmia neonatorum was an estimated 0.4 cases per 100,000 live births per year from 2013 to 2017.1–4 Gonococcal ophthalmia neonatorum can cause corneal scarring, ocular perforation, and blindness as early as 24 hours after birth.5–7 In the absence of ocular prophylaxis, transmission rates of gonococcal infection from mother to newborn are 30% to 50%.8
REAFFIRMATION
In 2011, the USPSTF reviewed the evidence on prophylactic ocular topical medication for all newborns to prevent gonococcal ophthalmia neonatorum and issued an A recommendation.5 The USPSTF has decided to use a reaffirmation deliberation process to update this recommendation. The USPSTF uses the reaffirmation process for well-established, evidence-based standards of practice in current primary care practice for which only a very high level of evidence would justify a change in the grade of the recommendation.9 In its deliberation of the evidence, the USPSTF considers whether the new evidence is of sufficient strength and quality to change its previous conclusions about the evidence.
BENEFITS OF PREVENTIVE MEDICATION
The USPSTF found convincing evidence that ocular prophylaxis of newborns with 0.5% erythromycin ophthalmic ointment can prevent gonococcal ophthalmia neonatorum.
HARMS OF PREVENTIVE MEDICATION
The USPSTF found convincing evidence that ocular prophylaxis of newborns with 0.5% erythromycin ophthalmic ointment is not associated with serious harms.
USPSTF ASSESSMENT
Using a reaffirmation process,9 the USPSTF concludes with high certainty that the net benefit of topical ocular prophylaxis of all newborns to prevent gonococcal ophthalmia neonatorum is substantial.
Clinical Considerations
PATIENT POPULATION UNDER CONSIDERATION
This recommendation applies to all newborns regardless of gestational age.
PREVENTIVE MEDICATION
Erythromycin ophthalmic ointment is considered effective in preventing gonococcal ophthalmia neonatorum.10 Other medications, such as tetracycline ophthalmic ointment and silver nitrate, have been evaluated for the prevention of gonococcal ophthalmia neonatorum but are no longer available in the United States.3 Gentamicin was used during a period of erythromycin shortage, although its use was associated with ocular reactions (chemical conjunctivitis).11 Povidone-iodine has been proposed for prophylaxis, but there are limited data on its benefits and harms.3 Currently, erythromycin is the only drug approved by the U.S. Food and Drug Administration for the prophylaxis of gonococcal ophthalmia neonatorum.11 Ocular prophylaxis of newborns is mandated in most states6 and is considered standard neonatal care.11
ADDITIONAL APPROACHES TO PREVENTION
The rates of gonococcal ophthalmia neonatorum are related to gonococcal infection rates in women of reproductive age.3 Accordingly, screening for and treatment of gonococcal infection in pregnant women is an important strategy for reducing the sexual transmission of gonorrhea and subsequent vertical transmission leading to gonococcal ophthalmia neonatorum. While screening and treatment programs have reduced the rates of gonorrhea in pregnant women, there are large disparities in access to prenatal care in the United States.1,12 Risk-based prophylaxis has also been proposed as an alternative strategy for preventing gonococcal ophthalmia neonatorum. Currently, there are no risk-based tools for screening pregnant women and no studies examining the use of risk-based vs. universal prophylaxis. Therefore, ocular prophylaxis remains an important tool in the prevention of gonococcal ophthalmia neonatorum.
USEFUL RESOURCES
The USPSTF recommends screening for gonorrhea in all sexually active women 24 years and younger and in older women at increased risk for infection, as well as pregnant women.13 The Centers for Disease Control and Prevention provides clinical guidance for ocular prophylaxis and treatment of gonococcal ophthalmia neonatorum.10
This recommendation statement was first published in JAMA. 2019;321(4):394-398.
The “Other Considerations,” “Discussion,” “Reaffirmation of Previous USPSTF Recommendation,” and “Recommendations of Others” sections of this recommendation statement are available at https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/ocular-prophylaxis-for-gonococcal-ophthalmia-neonatorum-preventive-medication1.
The USPSTF recommendations are independent of the U.S. government. They do not represent the views of the Agency for Healthcare Research and Quality, the U.S. Department of Health and Human Services, or the U.S. Public Health Service.
