Am Fam Physician. 2019;100(7):426-434
Author disclosure: No relevant financial affiliations.
Anticoagulation therapy is recommended for preventing, treating, and reducing the recurrence of venous thromboembolism, and preventing stroke in persons with atrial fibrillation. Direct oral anticoagulants are first-line agents for eligible patients for treating venous thromboembolism and preventing stroke in those with nonvalvular atrial fibrillation. Vitamin K antagonists are recommended for patients with mechanical valves and valvular atrial fibrillation. Vitamin K antagonists inhibit the production of vitamin K-related factors and require a minimum of five days overlap with parenteral anticoagulants, whereas direct oral anticoagulants directly inhibit factor II or factor Xa, providing more immediate anticoagulation. The immediate effect of direct oral anticoagulants permits select patients at low risk to initiate treatment in the outpatient setting for venous thromboembolism, including pulmonary embolism. Low-molecular-weight heparin continues to be recommended as a first-line treatment for patients with venous thromboembolism and active cancer, although there is growing evidence of effectiveness for the use of direct oral anticoagulants in this patient population. Validated bleeding risk assessments such as HAS-BLED should be performed at each visit and modifiable factors should be addressed. Major bleeding should be treated with vitamin K and 4-factor prothrombin complex concentrate for patients already being treated with a vitamin K antagonist. Idarucizumab has been effective for reversing the anticoagulant effects of dabigatran, and andexanet alfa has been effective for reversing the effects of rivaroxaban and apixaban.
Vitamin K antagonists (e.g., warfarin [Coumadin]), unfractionated heparin, low-molecular-weight heparin (LMWH), and direct oral anticoagulants are commonly used for the prevention and treatment of systemic embolism associated with atrial fibrillation, stroke, and venous thromboembolism (VTE). LMWH and select direct oral anticoagulants can be used for anticoagulation therapy initiation on an outpatient basis.
WHAT'S NEW ON THIS TOPIC
Compared with vitamin K antagonists, direct oral anticoagulants have fewer overall drug-drug interactions, a comparable (if not lower) bleeding rate, a shorter half-life, and fixed dosing based on indication, drug interactions, and renal or hepatic function.
American Academy of Family Physicians guidelines recommend the use of oral anticoagulants in patients with a CHADS2 score greater than 1 for the prevention of stroke in atrial fibrillation. The American Heart Association/American College of Cardiology/Heart Rhythm Society guidelines recommend a direct oral anticoagulant over a vitamin K antagonist, unless the patient has moderate-to-severe mitral stenosis or a mechanical heart valve.
In May 2018, andexanet alfa (Andexxa) was approved to reverse the anticoagulant effects of rivaroxaban (Xarelto) and apixaban (Eliquis) in patients with life-threatening or uncontrolled bleeding. Optimal dose, duration, need for repeat dosing, and mitigation of thromboembolic risk is yet to be delineated.
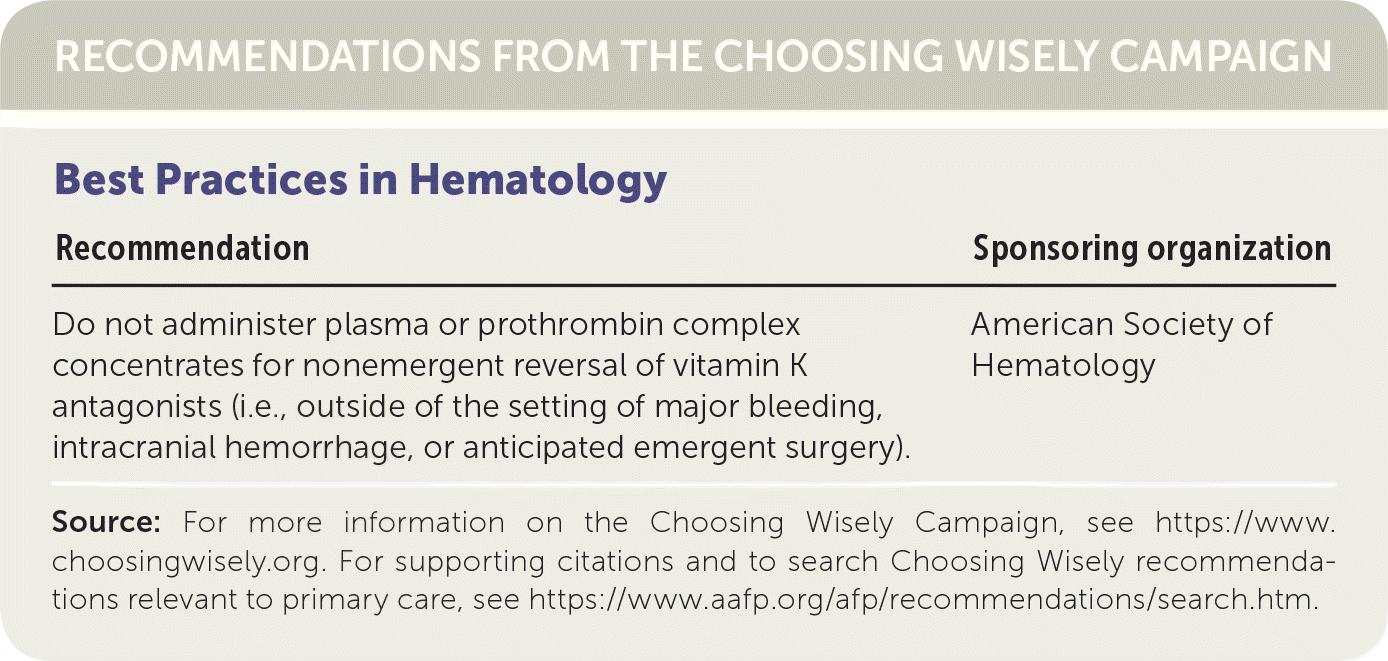
| Best Practices in Hematology | |
|---|---|
| Recommendation | Sponsoring organization |
| Do not administer plasma or prothrombin complex concentrates for nonemergent reversal of vitamin K antagonists (i.e., outside of the setting of major bleeding, intracranial hemorrhage, or anticipated emergent surgery). | American Society of Hematology |
This article focuses on the indications for anticoagulation therapy, direct oral anticoagulant therapy, and recommendations from guidelines. Most of the recommendations are based on the 10th edition of the American College of Chest Physicians (ACCP) guidelines on antithrombotic therapy for VTE disease (Table 1).1–5
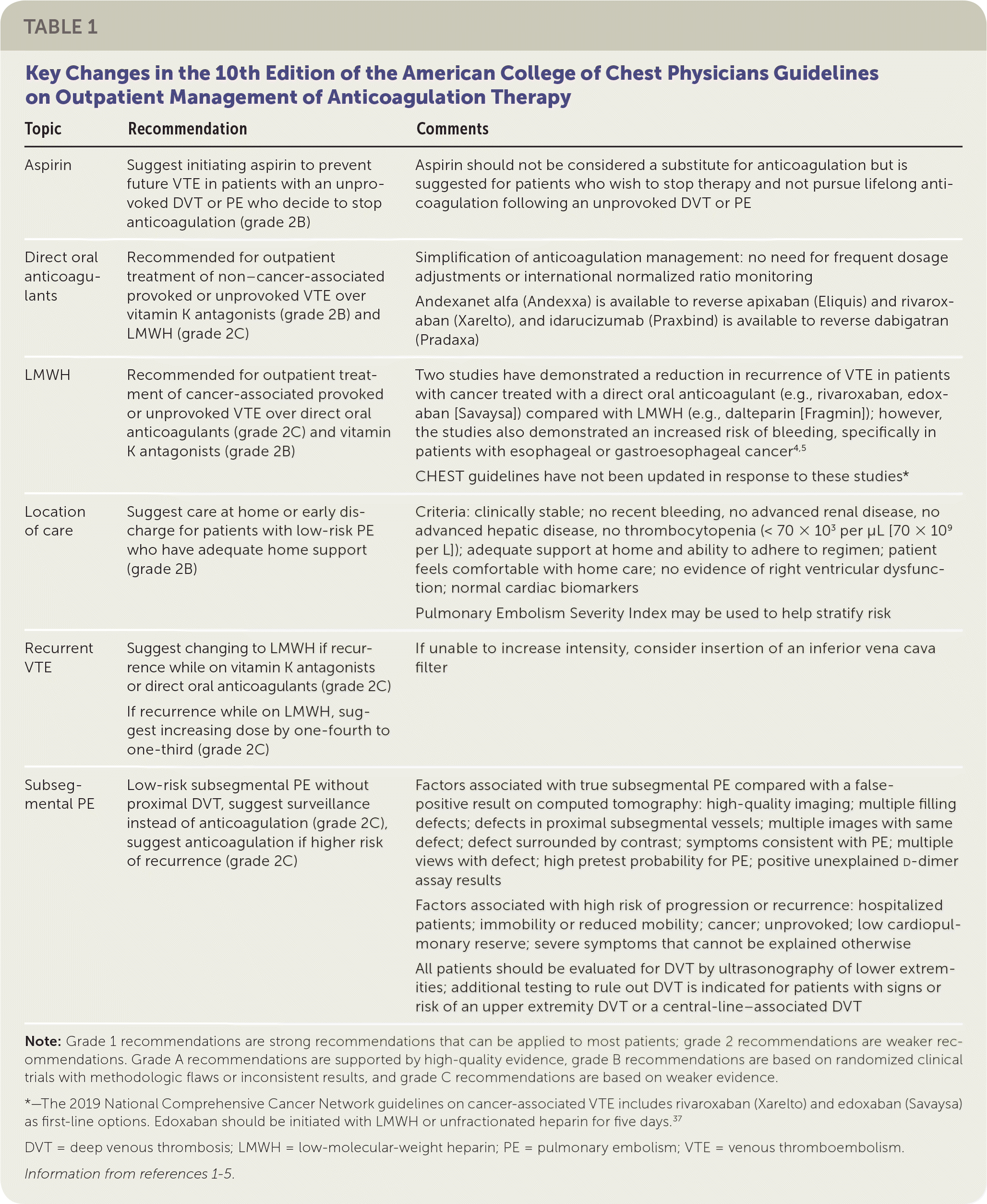
| Topic | Recommendation | Comments |
|---|---|---|
| Aspirin | Suggest initiating aspirin to prevent future VTE in patients with an unprovoked DVT or PE who decide to stop anticoagulation (grade 2B) | Aspirin should not be considered a substitute for anticoagulation but is suggested for patients who wish to stop therapy and not pursue lifelong anticoagulation following an unprovoked DVT or PE |
| Direct oral anticoagulants | Recommended for outpatient treatment of non–cancer-associated provoked or unprovoked VTE over vitamin K antagonists (grade 2B) and LMWH (grade 2C) | Simplification of anticoagulation management: no need for frequent dosage adjustments or international normalized ratio monitoring Andexanet alfa (Andexxa) is available to reverse apixaban (Eliquis) and rivaroxaban (Xarelto), and idarucizumab (Praxbind) is available to reverse dabigatran (Pradaxa) |
| LMWH | Recommended for outpatient treatment of cancer-associated provoked or unprovoked VTE over direct oral anticoagulants (grade 2C) and vitamin K antagonists (grade 2B) | Two studies have demonstrated a reduction in recurrence of VTE in patients with cancer treated with a direct oral anticoagulant (e.g., rivaroxaban, edoxaban [Savaysa]) compared with LMWH (e.g., dalteparin [Fragmin]); however, the studies also demonstrated an increased risk of bleeding, specifically in patients with esophageal or gastroesophageal cancer 4,5 CHEST guidelines have not been updated in response to these studies* |
| Location of care | Suggest care at home or early discharge for patients with low-risk PE who have adequate home support (grade 2B) | Criteria: clinically stable; no recent bleeding, no advanced renal disease, no advanced hepatic disease, no thrombocytopenia (< 70 × 103 per μL [70 × 109 per L]); adequate support at home and ability to adhere to regimen; patient feels comfortable with home care; no evidence of right ventricular dysfunction; normal cardiac biomarkers Pulmonary Embolism Severity Index may be used to help stratify risk |
| Recurrent VTE | Suggest changing to LMWH if recurrence while on vitamin K antagonists or direct oral anticoagulants (grade 2C) If recurrence while on LMWH, suggest increasing dose by one-fourth to one-third (grade 2C) | If unable to increase intensity, consider insertion of an inferior vena cava filter |
| Subsegmental PE | Low-risk subsegmental PE without proximal DVT, suggest surveillance instead of anticoagulation (grade 2C), suggest anticoagulation if higher risk of recurrence (grade 2C) | Factors associated with true subsegmental PE compared with a false-positive result on computed tomography: high-quality imaging; multiple filling defects; defects in proximal subsegmental vessels; multiple images with same defect; defect surrounded by contrast; symptoms consistent with PE; multiple views with defect; high pretest probability for PE; positive unexplained d-dimer assay results Factors associated with high risk of progression or recurrence: hospitalized patients; immobility or reduced mobility; cancer; unprovoked; low cardiopulmonary reserve; severe symptoms that cannot be explained otherwise All patients should be evaluated for DVT by ultrasonography of lower extremities; additional testing to rule out DVT is indicated for patients with signs or risk of an upper extremity DVT or a central-line–associated DVT |
Medication Overview
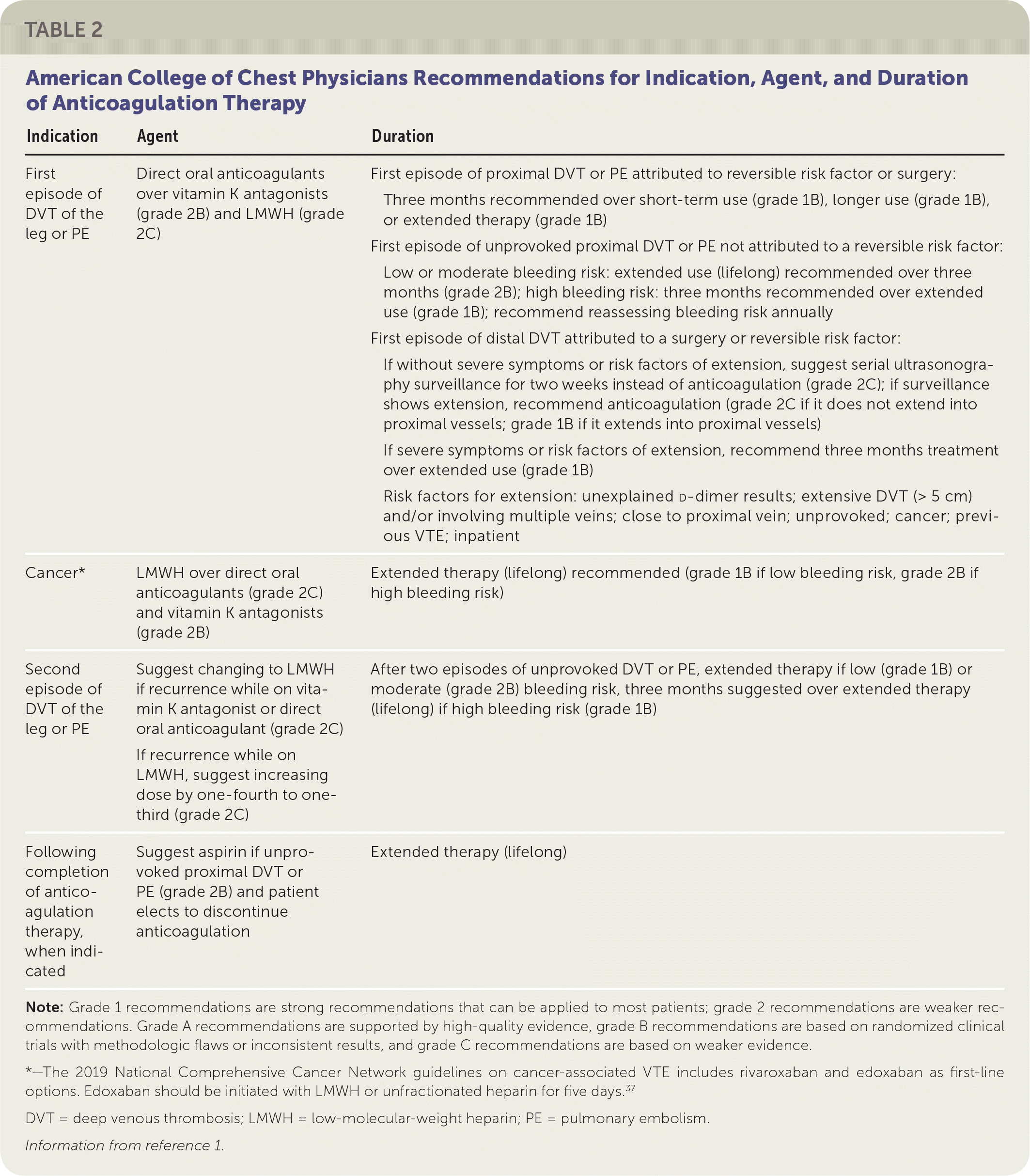
| Indication | Agent | Duration |
|---|---|---|
| First episode of DVT of the leg or PE | Direct oral anticoagulants over vitamin K antagonists (grade 2B) and LMWH (grade 2C) |
|
| Cancer* | LMWH over direct oral anticoagulants (grade 2C) and vitamin K antagonists (grade 2B) | Extended therapy (lifelong) recommended (grade 1B if low bleeding risk, grade 2B if high bleeding risk) |
| Second episode of DVT of the leg or PE | Suggest changing to LMWH if recurrence while on vitamin K antagonist or direct oral anticoagulant (grade 2C) If recurrence while on LMWH, suggest increasing dose by one-fourth to one-third (grade 2C) | After two episodes of unprovoked DVT or PE, extended therapy if low (grade 1B) or moderate (grade 2B) bleeding risk, three months suggested over extended therapy (lifelong) if high bleeding risk (grade 1B) |
| Following completion of anticoagulation therapy, when indicated | Suggest aspirin if unprovoked proximal DVT or PE (grade 2B) and patient elects to discontinue anticoagulation | Extended therapy (lifelong) |
VITAMIN K ANTAGONISTS
In the liver, vitamin K antagonists inhibit the cyclic interconversion of vitamin K, indirectly reducing clotting and synthesis for factors II, VII, IX, and X. Vitamin K antagonists also decrease levels of vitamin K–dependent anticoagulation proteins C and S; therefore, carboxylation inhibition can result in a paradoxical increased clotting risk when vitamin K antagonist therapy is initiated. Anticoagulant effects are delayed for five days after changes to dosing, including therapy initiation, because of the variable half-lives of previously formed circulating clotting factors.4
Dosing. Patients receiving vitamin K antagonist therapy should be treated using a systematic process to optimize effectiveness and minimize adverse effects. Heparin or LMWH should be administered at initiation of the vitamin K antagonist and be continued for a minimum of five days and until the international normalized ratio (INR) is in the targeted therapeutic range for a minimum of 24 hours. The targeted INR range depends on indication for use and, at times, patient comorbidities.
For most patients, vitamin K antagonists should be initiated at a maintenance dosage of 5 mg per day. Older patients and persons with liver disease, poor nutritional status, or heart failure may require lower initiation dosages.4 Diarrhea, fever, and hyperthyroidism can also potentiate the effect of vitamin K antagonists. Genetic factors can predispose patients to reduced vitamin K antagonist requirements, as well as resistance. Although there is a small subset of patients who may have unexpected responses to vitamin K antagonists, it is not currently recommended that patients undergo genetic testing.4
After a baseline INR is determined, the next INR should be obtained after the patient has received two or three doses of the vitamin K antagonist. Monitoring should then be decreased to twice weekly until the INR is within the therapeutic range, then decreased to weekly, every other week, and finally monthly.4 The ACCP guidelines recommend INR monitoring once every 12 weeks for patients who are stable (defined as at least three months of consistent results with no required adjustment of vitamin K antagonist dosing).
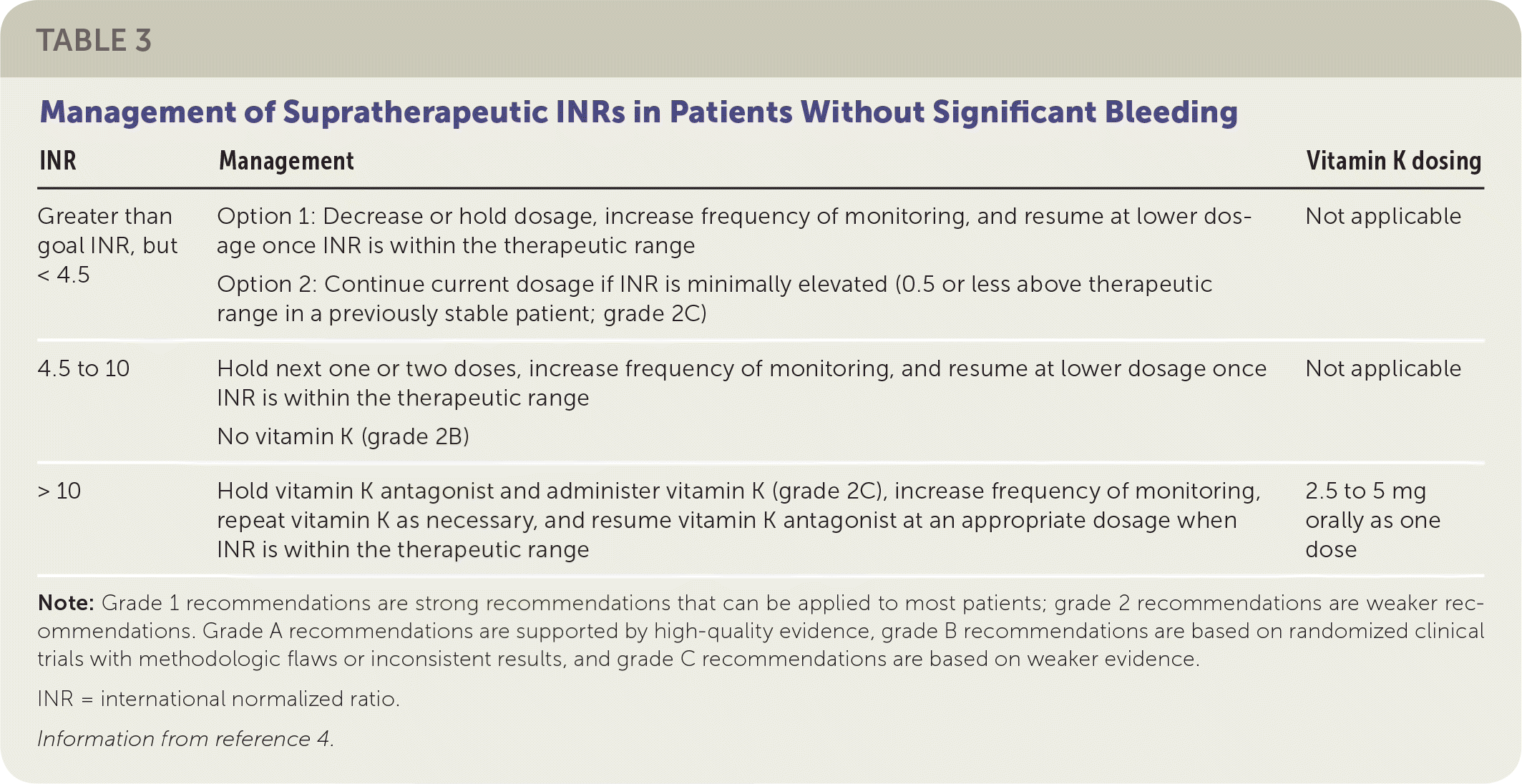
| INR | Management | Vitamin K dosing |
|---|---|---|
| Greater than goal INR, but < 4.5 | Option 1: Decrease or hold dosage, increase frequency of monitoring, and resume at lower dosage once INR is within the therapeutic range Option 2: Continue current dosage if INR is minimally elevated (0.5 or less above therapeutic range in a previously stable patient; grade 2C) | Not applicable |
| 4.5 to 10 | Hold next one or two doses, increase frequency of monitoring, and resume at lower dosage once INR is within the therapeutic range No vitamin K (grade 2B) | Not applicable |
| > 10 | Hold vitamin K antagonist and administer vitamin K (grade 2C), increase frequency of monitoring, repeat vitamin K as necessary, and resume vitamin K antagonist at an appropriate dosage when INR is within the therapeutic range | 2.5 to 5 mg orally as one dose |
Vitamin K antagonists should be taken at the same time every day. An advantage to evening administration is the ability to adjust or hold the dose the same day that the INR result becomes available. If the INR is not within the desired therapeutic range after excluding explanatory factors, a 5% to 20% increase or decrease in the total weekly dosage is recommended.6,7 Patients should be provided with the simplest regimen to achieve the new total weekly dosage.
Drug and Food Interactions. Vitamin K antagonists are subject to many drug interactions. Select drug-drug interactions that are considered to potentiate or inhibit the effects of vitamin K antagonists are listed in Table 4.8 When an interacting drug is initiated or discontinued, more frequent INR checks are recommended. The timing depends on the type of interaction, what the interacting drug is, and the clinical judgement of the physician.
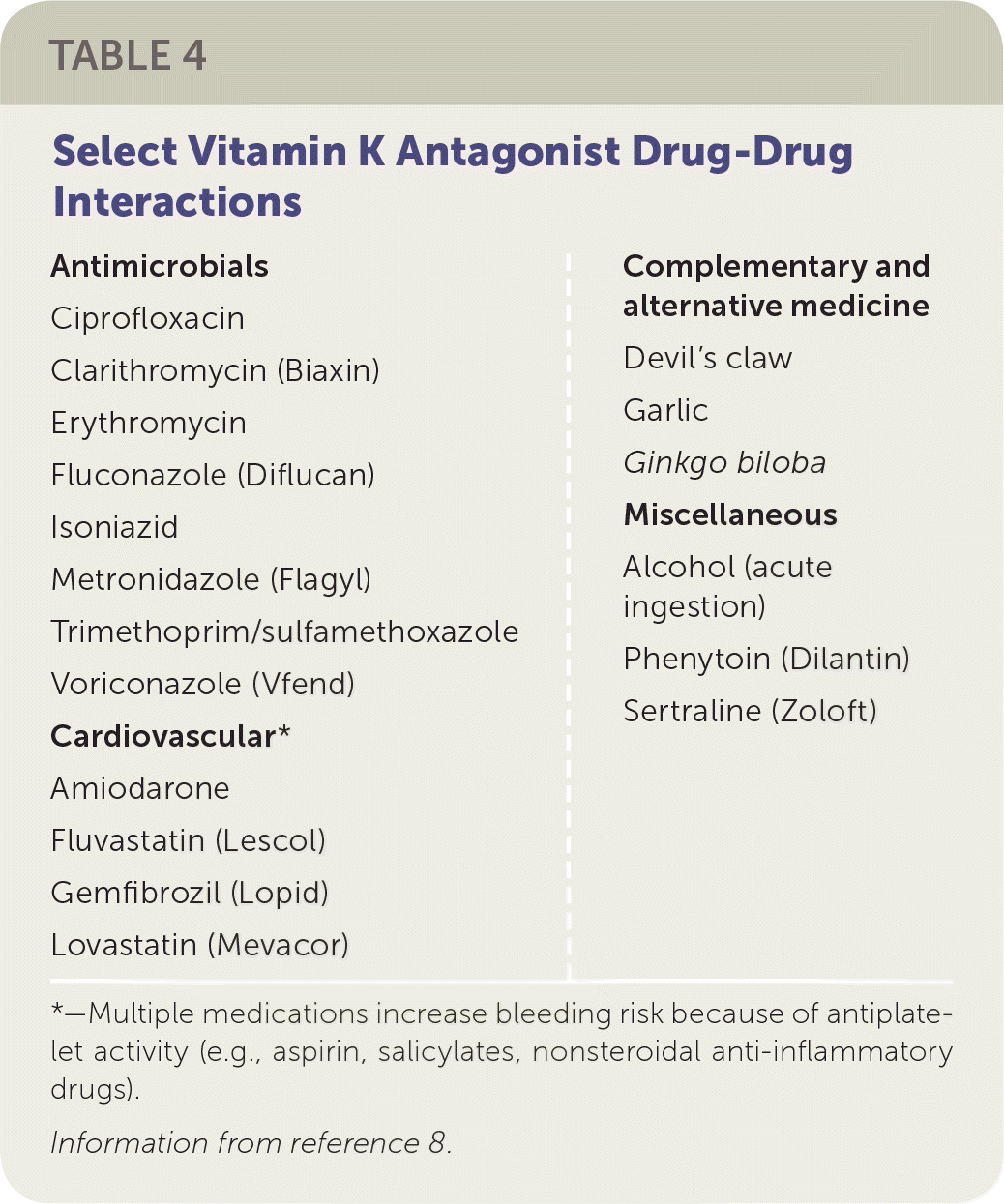
| Antimicrobials Ciprofloxacin Clarithromycin (Biaxin) Erythromycin Fluconazole (Diflucan) Isoniazid Metronidazole (Flagyl) Trimethoprim/sulfamethoxazole Voriconazole (Vfend) Cardiovascular* Amiodarone Fluvastatin (Lescol) Gemfibrozil (Lopid) Lovastatin (Mevacor) Complementary and alternative medicine Devil's claw Garlic Ginkgo biloba Miscellaneous Alcohol (acute ingestion) Phenytoin (Dilantin) Sertraline (Zoloft) |
Foods with high vitamin K concentrations, such as leafy green vegetables, have the potential to partially reverse anticoagulation effects of the vitamin K antagonist.4 A consistent diet is more important than limiting dietary vitamin K.
LOW-MOLECULAR-WEIGHT HEPARIN
Considerations for parenteral medications are provided in eTable A. Dalteparin (Fragmin) and enoxaparin (Lovenox) are commonly used LMWHs in clinical practice. LMWH is derived from unfractionated heparin and has an increased affinity for factor Xa relative to thrombin.4 LMWH's anticoagulant effect is primarily from factor Xa inhibition because of its smaller size and its lessened ability to inactivate thrombin compared with unfractionated heparin. Subcutaneous LMWH has a predictable absorption and degree of anticoagulation, so monitoring with anti–factor Xa levels is not routinely recommended. Although LMWH has a similar bleeding risk and lower heparin-induced thrombocytopenia risk compared with unfractionated heparin, a patient with a history of heparin-induced thrombocytopenia should not take LMWH.1
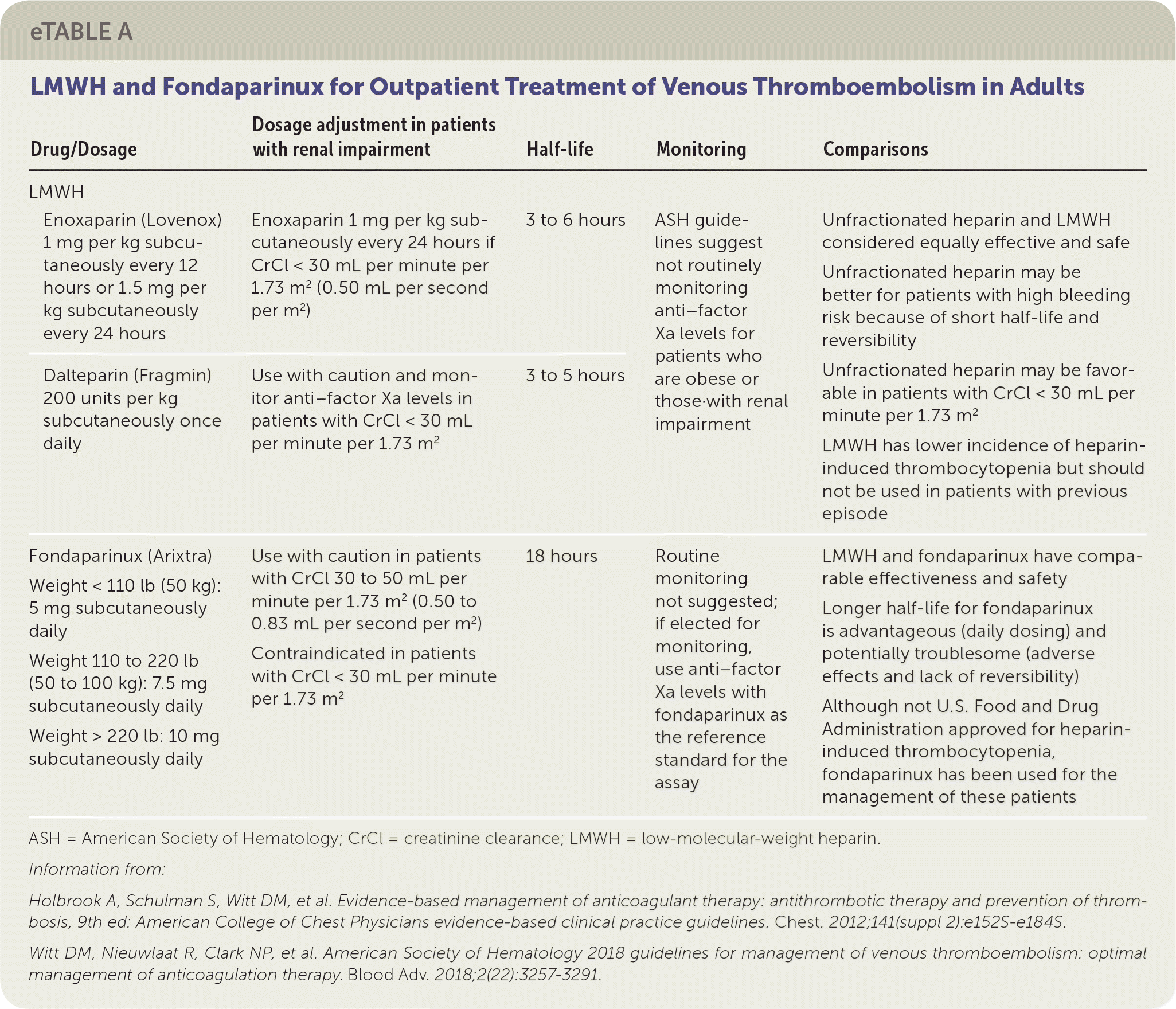
| Drug/Dosage | Dosage adjustment in patients with renal impairment | Half-life | Monitoring | Comparisons | |
|---|---|---|---|---|---|
| LMWH | |||||
| Enoxaparin (Lovenox) 1 mg per kg subcutaneously every 12 hours or 1.5 mg per kg subcutaneously every 24 hours | Enoxaparin 1 mg per kg subcutaneously every 24 hours if CrCl < 30 mL per minute per 1.73 m2 (0.50 mL per second per m2) | 3 to 6 hours | ASH guidelines suggest not routinely monitoring anti–factor Xa levels forpatients who are obese or those with renal impairment | Unfractionated heparin and LMWH considered equally effective and safe Unfractionated heparin may be better for patients with high bleeding risk because of short half-life and reversibility Unfractionated heparin may be favorable in patients with CrCl < 30 mL per minute per 1.73 m2 LMWH has lower incidence of heparin-induced thrombocytopenia but should not be used in patients with previous episode | |
| Dalteparin (Fragmin) 200 units per kg subcutaneously once daily | Use with caution and monitor anti–factor Xa levels in patients with CrCl < 30 mL per minute per 1.73 m2 | 3 to 5 hours | ASH guidelines suggest not routinely monitoring anti–factor Xa levels forpatients who are obese or those with renal impairment | ||
| Fondaparinux (Arixtra) Weight < 110 lb (50 kg): 5 mg subcutaneously daily Weight 110 to 220 lb (50 to 100 kg): 7.5 mg subcutaneously daily Weight > 220 lb: 10 mg subcutaneously daily | Use with caution in patients with CrCl 30 to 50 mL per minute per 1.73 m2 (0.50 to 0.83 mL per second per m2) Contraindicated in patients with CrCl < 30 mL per minute per 1.73 m2 | 18 hours | Routine monitoring not suggested; if elected for monitoring, use anti–factor Xa levels with fondaparinux as the reference standard for the assay | LMWH and fondaparinux have comparable effectiveness and safety Longer half-life for fondaparinux is advantageous (daily dosing) and potentially troublesome (adverse effects and lack of reversibility) Although not U.S. Food and Drug Administration approved for heparin-induced thrombocytopenia, fondaparinux has been used for the management of these patients | |
DIRECT ORAL ANTICOAGULANTS
Warfarin was approved in 1954, and no other oral option existed for patients requiring long-term anticoagulation therapy until 2010 when the direct thrombin inhibitor dabigatran (Pradaxa) was approved. Since dabigatran's approval, four additional direct oral factor Xa inhibitors have been approved. Characteristics of these anticoagulants are provided in Table 59–13 and eTable B. Physicians should not automatically consider all patients taking vitamin K antagonists to be good candidates for direct oral anticoagulants because of the diversity in the characteristics of these medications.
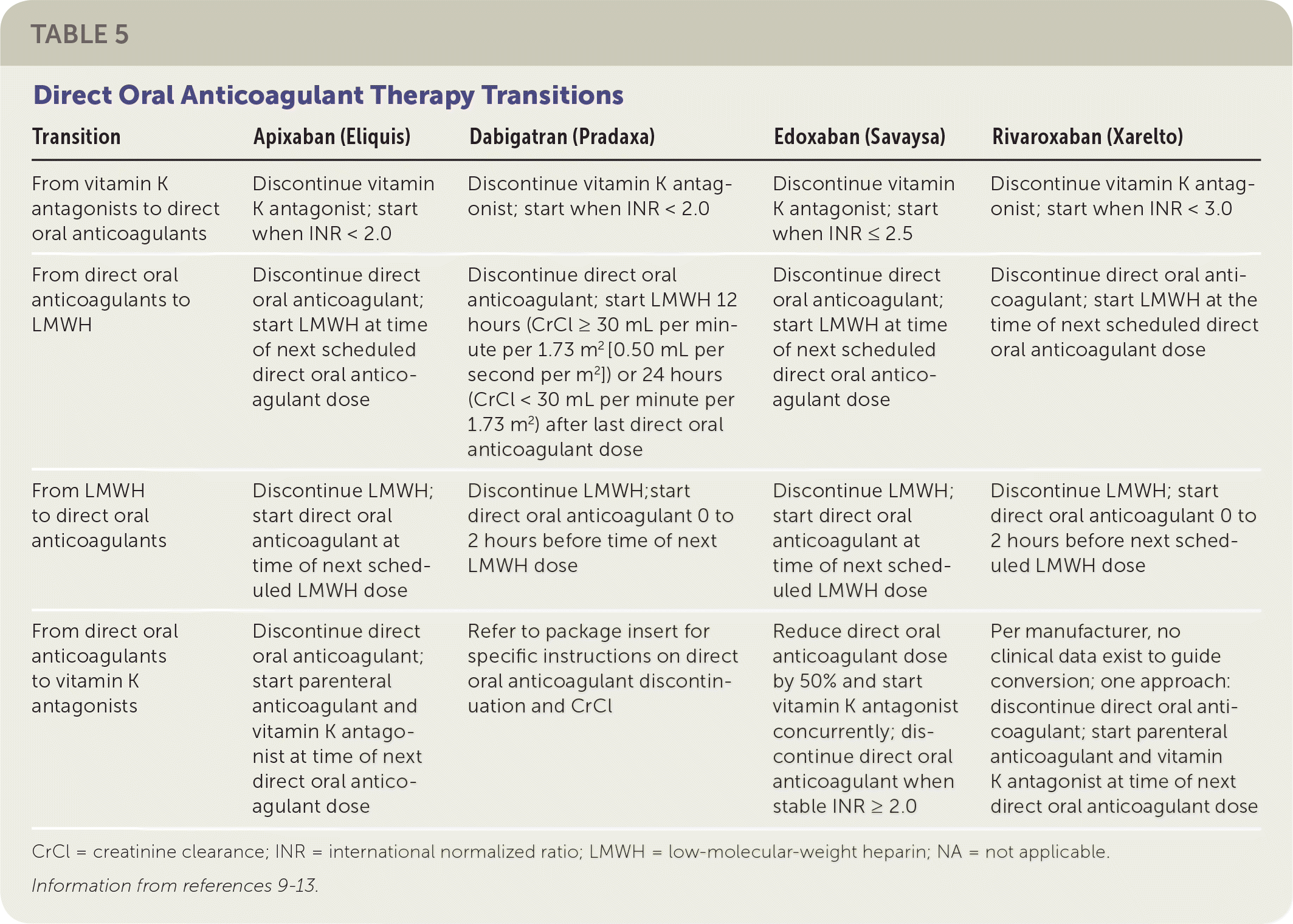
| Transition | Apixaban (Eliquis) | Dabigatran (Pradaxa) | Edoxaban (Savaysa) | Rivaroxaban (Xarelto) |
|---|---|---|---|---|
| From vitamin K antagonists to direct oral anticoagulants | Discontinue vitamin K antagonist; start when INR < 2.0 | Discontinue vitamin K antagonist; start when INR < 2.0 | Discontinue vitamin K antagonist; start when INR ≤ 2.5 | Discontinue vitamin K antagonist; start when INR < 3.0 |
| From direct oral anticoagulants to LMWH | Discontinue direct oral anticoagulant; start LMWH at time of next scheduled direct oral anticoagulant dose | Discontinue direct oral anticoagulant; start LMWH 12 hours (CrCl ≥ 30 mL per minute per 1.73 m2 [0.50 mL per second per m2]) or 24 hours (CrCl < 30 mL per minute per 1.73 m2) after last direct oral anticoagulant dose | Discontinue direct oral anticoagulant; start LMWH at time of next scheduled direct oral anticoagulant dose | Discontinue direct oral anticoagulant; start LMWH at the time of next scheduled direct oral anticoagulant dose |
| From LMWH to direct oral anticoagulants | Discontinue LMWH; start direct oral anticoagulant at time of next scheduled LMWH dose | Discontinue LMWH;start direct oral anticoagulant 0 to 2 hours before time of next LMWH dose | Discontinue LMWH; start direct oral anticoagulant at time of next scheduled LMWH dose | Discontinue LMWH; start direct oral anticoagulant 0 to 2 hours before next scheduled LMWH dose |
| From direct oral anticoagulants to vitamin K antagonists | Discontinue direct oral anticoagulant; start parenteral anticoagulant and vitamin K antagonist at time of next direct oral anticoagulant dose | Refer to package insert for specific instructions on direct oral anticoagulant discontinuation and CrCl | Reduce direct oral anticoagulant dose by 50% and start vitamin K antagonist concurrently; discontinue direct oral anticoagulant when stable INR ≥ 2.0 | Per manufacturer, no clinical data exist to guide conversion; one approach: discontinue direct oral anticoagulant; start parenteral anticoagulant and vitamin K antagonist at time of next direct oral anticoagulant dose |
| Drug | Indications | Usual dosage (assess renal function before beginning direct oral anticoagulant and as clinically indicated)* | Hepatic dosing | Impact of weight† | Half-life | Cost‡ | Comments |
|---|---|---|---|---|---|---|---|
| Apixaban (Eliquis) | Reduce risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation; treat DVT and PE Not recommended as an acute alternative to unfractionated heparin in patients with PE who present with hemodynamic instability or may receive thrombolysis or pulmonary embolectomy Reduce risk of recurrent DVT and PE and DVT prophylaxis (hip and knee replacement) | Prophylaxis for stroke and systemic embolism in nonvalvular atrial fibrillation:
| Child-Pugh Class A: do not need dose adjustment Child-Pugh Class B and C: not recommended | Refer to usual dosage section for impact of lower weight Appropriate standard direct oral anticoagulant dosing in patients with a BMI ≤ 40 kg per m2 or weight ≤ 120 kg (265 lb) Suggest direct oral anticoagulants not be used in patients with a BMI > 40 kg per m2 or weight > 120 kg; if used in these patients, check drug-specific peak and trough levels | 12 hours | $460 for 60 5-mg tablets | Starter pack for initial dosing for treatment of DVT and PE Coupons available for starter pack and maintenance dosing |
| Betrixaban (Bevyxxa) | VTE prophylaxis in adults hospitalized for an acute medical illness | VTE prophylaxis in at-risk, acutely ill, hospitalized patients: 160 mg with food for first dose, then 80 mg per day with food for 35 to 42 days CrCl 15 to 29 mL per minute per 1.73 m2 (0.25 to 0.48 mL per second per m2): 80 mg with food for first dose, then 40 mg per day with food for 35 to 42 days With P-glycoprotein inhibitors:
| Not recommended in patients with hepatic impairment | Appropriate standard direct oral anticoagulant dosing in patients with a BMI ≥ 40 kg per m2 or weight ≥ 120 kg Suggest direct oral anticoagulants not be used in patients with a BMI > 40 kg per m2 or weight > 120 kg; if used in these patients, check drug-specific peak and trough levels | 19 to 27 hours | $470 for 30 80-mg capsules | Take with food when used for VTE prophylaxis |
| Dabigatran (Pradaxa) | Reduce risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation; treat DVT and PE Not recommended as an acute alternative to unfractionated heparin in patients with PE who present with hemodynamic instability or may receive thrombolysis or pulmonary embolectomy Reduce risk of recurrent DVT and PE, and DVT prophylaxis (hip replacement) | Prophylaxis for stroke and systemic embolism in nonvalvular atrial fibrillation:
Prophylaxis for DVT or PE:
Reduce recurrence:
DVT or PE treatment:
| Limited data in patients with hepatic impairment; no specific dosing adjustment recommended | Appropriate standard direct oral anticoagulant dosing in patients with a BMI ≤ 40 kg per m2 or weight ≤ 120 kg Suggest direct oral anticoagulants not be used in patients with a BMI > 40 kg per m2 or weight > 120 kg; if used in these patients, check drug-specific peak and trough levels | 12 to 17 hours | $420 for 60 150-mg capsules | Do not chew, break, or open capsules Capsules must be dispensed in original container and cannot be repackaged because of the sensitivity to moisture May cause dyspepsia |
| Edoxaban (Savaysa) | Reduce risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation; treat DVT and PE Not recommended as an acute alternative to unfractionated heparin in patients with PE who present with hemodynamic instability or may receive thrombolysis or pulmonary embolectomy | Prophylaxis for stroke and systemic embolism in nonvalvular atrial fibrillation:
DVT or PE treatment:
| Child-Pugh Class A: do not need dose adjustment Child-Pugh Class B and C: not recommended | Refer to usual dosage section for impact of lower weight Appropriate standard direct oral anticoagulant dosing in patients with a BMI ≤ 40 kg per m2 or weight ≤ 120 kg Suggest direct oral anticoagulants not be used in patients with a BMI > 40 kg per m2 or weight > 120 kg; if used in these patients, check drug-specific peak and trough levels | 10 to 14 hours | $380 for 30 60-mg tablets | — |
| Rivaroxaban (Xarelto) | Reduce risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation; treat DVT and PE Not recommended as an acute alternative to unfractionated heparin in patients with PE who present with hemodynamic instability or may receive thrombolysis or pulmonary embolectomy Reduce risk of recurrent DVT and PE, and for DVT prophylaxis (hip and knee replacement) | Discontinue in patients who develop acute renal failure on rivaroxaban Prophylaxis for stroke and systemic embolism in nonvalvular atrial fibrillation:
Prophylaxis for DVT or PE:
Reduce recurrence:
DVT or PE treatment:
| Child-Pugh Class A: do not need dose adjustment Child-Pugh Class B and C: not recommended | Appropriate standard direct oral anticoagulant dosing in patients with a BMI ≤ 40 kg per m2 or weight ≤ 120 kg Suggest direct oral anticoagulants not be used In patients with a BMI > 40 kg per m2 or weight > 120 kg; if used in these patients, check drug-specific peak and trough levels | 5 to 9 hours | $470 for 30 20-mg tablets | Take with food Starter pack for initial dosing for treatment of DVT and PE Coupons available for starter pack and maintenance dosing Information |
Direct oral anticoagulants are first-line agents for eligible patients for the treatment of VTE and prevention of stroke in patients with nonvalvular atrial fibrillation. Compared with vitamin K antagonists, direct oral anticoagulants have the advantage of not requiring direct monitoring, having minimal drug-food interactions, and having a quicker onset of action to therapeutic effect. Direct oral anticoagulants have fewer overall drug-drug interactions (Table 65,9–13); a comparable (if not lower) bleeding rate; a shorter half-life; and fixed dosing based on indication, drug interactions, and renal or hepatic function. Among the direct oral anticoagulants, there are key differences including the need for parenteral anticoagulation lead-in, once or twice per day dosing, and degree of renal excretion. Of the direct oral anticoagulants, dabigatran and edoxaban (Savaysa) have the highest renal elimination (approximately 80% and 50%, respectively) and should be used with caution in patients with renal impairment.14 Each of the direct oral anticoagulants has a risk of bleeding. Compared with vitamin K antagonists and with other direct oral anticoagulants, apixaban (Eliquis) has less major bleeding. In addition, rivaroxaban (Xarelto) and dabigatran have a higher risk of gastrointestinal bleeding compared with apixaban.15 Real world experience with direct oral anticoagulants in terms of safety and effectiveness seems consistent with published trials.15,16
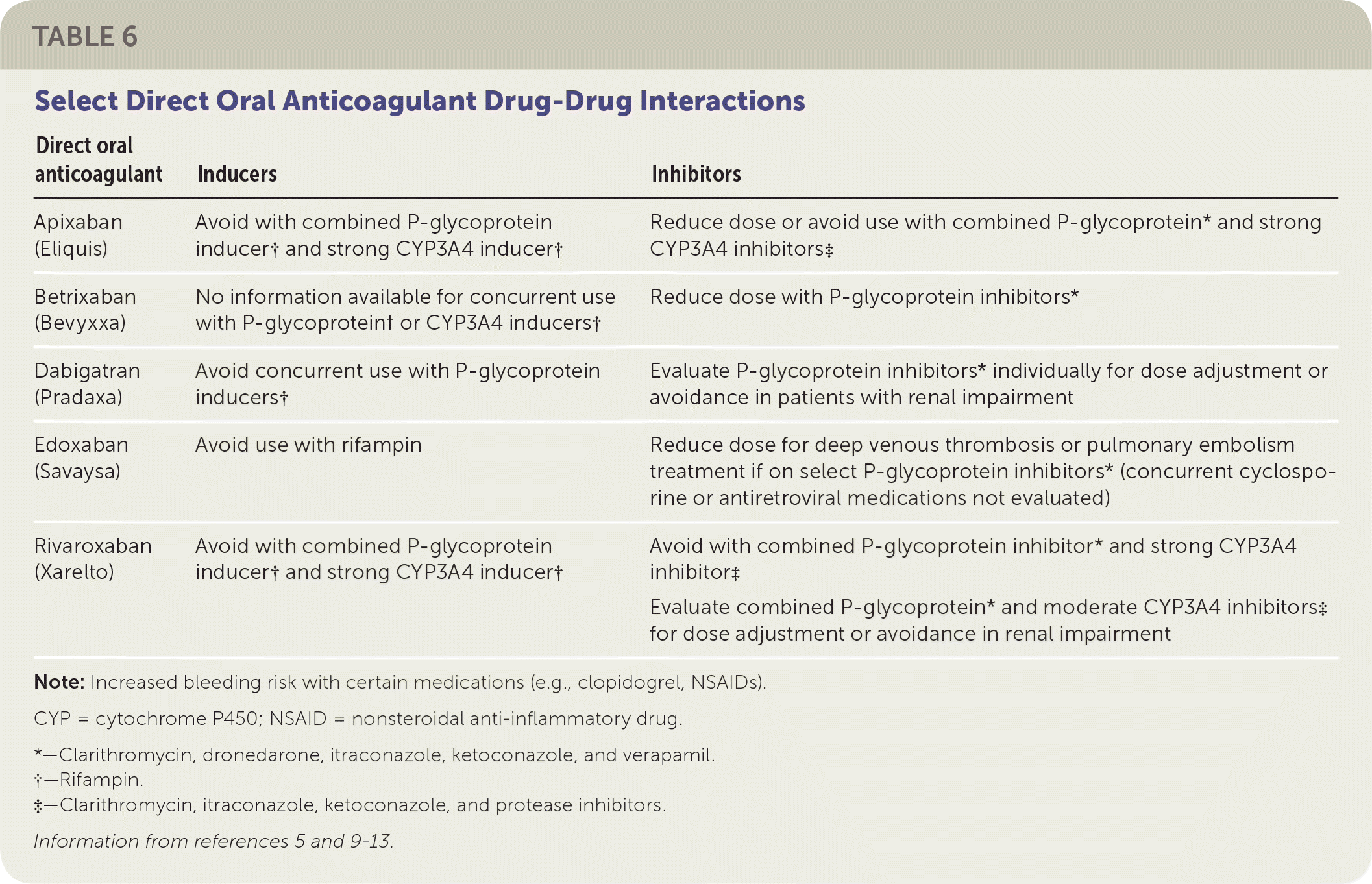
| Direct oral anticoagulant | Inducers | Inhibitors |
|---|---|---|
| Apixaban (Eliquis) | Avoid with combined P-glycoprotein inducer† and strong CYP3A4 inducer† | Reduce dose or avoid use with combined P-glycoprotein* and strong CYP3A4 inhibitors‡ |
| Betrixaban (Bevyxxa) | No information available for concurrent use with P-glycoprotein† or CYP3A4 inducers† | Reduce dose with P-glycoprotein inhibitors* |
| Dabigatran (Pradaxa) | Avoid concurrent use with P-glycoprotein inducers† | Evaluate P-glycoprotein inhibitors* individually for dose adjustment or avoidance in patients with renal impairment |
| Edoxaban (Savaysa) | Avoid use with rifampin | Reduce dose for deep venous thrombosis or pulmonary embolism treatment if on select P-glycoprotein inhibitors* (concurrent cyclosporine or antiretroviral medications not evaluated) |
| Rivaroxaban (Xarelto) | Avoid with combined P-glycoprotein inducer† and strong CYP3A4 inducer† | Avoid with combined P-glycoprotein inhibitor* and strong CYP3A4 inhibitor‡ Evaluate combined P-glycoprotein* and moderate CYP3A4 inhibitors‡ for dose adjustment or avoidance in renal impairment |
Stroke Prevention in Atrial Fibrillation
In patients who have had acute ischemic stroke, the prevalence of comorbid atrial fibrillation is increasing, and atrial fibrillation–associated strokes have a higher mortality rate.17 One study found that treatment decisions are often not guideline adherent.18 The CHADS2 (congestive heart failure; hypertension; age 75 years or older; diabetes mellitus; prior stroke, transient ischemic attack, or thromboembolism [doubled]) tool (https://www.mdcalc.com/chads2-score-atrial-fibrillation-stroke-risk) or CHA2DS2-VASc (congestive heart failure; hypertension; age 75 years or older [doubled]; diabetes mellitus; prior stroke, transient ischemic attack, or thromboembolism [doubled]; vascular disease; age 65 to 74 years; sex category) tool (https://www.mdcalc.com/cha2ds2-vasc-score-atrial-fibrillation-stroke-risk) can be used to estimate the risk of stroke. American Academy of Family Physicians (AAFP) guidelines recommend the use of oral anticoagulants with a CHADS2 score higher than 1.19 These guidelines also allow for anticoagulation in patients with a CHADS2 score of 1 in certain circumstances.19 The recently published American Heart Association (AHA) /American College of Cardiology (ACC)/Heart Rhythm Society (HRS) guidelines recommend a direct oral anticoagulant over vitamin K antagonists, unless the patient has moderate-to-severe mitral stenosis or a mechanical heart valve.20 Anticoagulation is recommended for male patients with a CHA2DS2-VASc score of 2 or higher and female patients with a CHA2DS2-VASc score of 3 or higher. AHA/ACC/HRS guidelines,20 and the recently published ACCP guidelines attempt to further specify recommendations for low-risk patients as defined by low CHA2DS2-VASc scores.21
According to these recommendations, a CHA2DS2-VASc score as low as 1 for men and 2 for women warrants consideration for anticoagulation therapy.21 Guidelines also recommend that if a therapeutic INR range of 2 to 3 cannot be attained more than 70% of the time, then consideration should be given to changing the treatment to a direct oral anticoagulant. Direct oral anticoagulants or vitamin K antagonists can also be used for the periods before and after cardioversion. Compared with vitamin K antagonists, direct oral anticoagulants are associated with a reduction in the incidence of stroke of 21% to 35% and a reduction in the incidence of intracranial hemorrhage of 33% to 60%.22–25
One comparative effectiveness analysis looked at the treatment of patients with atrial fibrillation who may not have been well-represented in clinical trials because of multiple comorbidities.26 This study used Medicare data to compare vitamin K antagonists with dabigatran and rivaroxaban in patients with atrial fibrillation and multiple chronic conditions. The study found that effectiveness was similar for all oral anticoagulants and risk of major hemorrhage was reduced among dabigatran users when compared with rivaroxaban (but not vitamin K antagonists) in patients with a moderate-to-high burden of chronic conditions.26 Rates of gastrointestinal hemorrhage were highest in the rivaroxaban group. Finally, in this vulnerable population, the relative hazard of death rates was generally lower for rivaroxaban and dabigatran compared with vitamin K antagonists.26
Bleeding Risk Assessment
Multiple risk assessment scoring systems have been developed, with inconsistent success in predicting major bleeding event incidence.27 The ACCP guidelines recommend assessing bleeding risk for patients with VTE or atrial fibrillation as an essential step to guiding treatment decisions such as the duration of treatment. ACCP risk factors for VTE (e.g., advanced age, cancer, renal or hepatic failure) and an associated scoring system to categorize low (no risk factors), moderate (one risk factor), and high (two or more risk factors) risk should be used to determine treatment decisions.1
The ACCP and AAFP recommend using the HAS-BLED (hypertension, abnormal renal function and liver function, stroke, bleeding, labile INR, elderly [older than 65 years], drugs and alcohol) scoring tool (https://www.mdcalc.com/has-bled-score-major-bleeding-risk) to assess risk of bleeding for patients with atrial fibrillation.19,21 Because of the overlap in risk of ischemic stroke and bleeding, patients with the highest risk of ischemic stroke will commonly also have high bleeding risk. Bleeding risk in patients at high risk for ischemic stroke should rarely be used as a reason to withhold anticoagulation for patients with atrial fibrillation.21 Risk should be evaluated at each visit and modifiable risk factors, such as alcohol consumption, anemia, anticoagulation control, and use of medications that increase risk of bleeding such as aspirin and nonsteroidal anti-inflammatory drugs, should be addressed.21
Management of Bleeding
The ACC published an expert consensus decision pathway in 2017 on the management of bleeding for patients taking oral anticoagulants.28 Management of bleeding for patients taking vitamin K antagonists depends on the severity of the bleed. For major bleeding (defined as all bleeds associated with hemodynamic compromise, occurring in an anatomically critical site, associated with a decrease of hemoglobin of 2 g per dL [20 g per L] or greater, or requiring transfusion of two units of packed red blood cells or more), the patient should receive supportive care, 4-factor prothrombin complex concentrate, and intravenous vitamin K. The only available 4-factor prothrombin complex concentrate is Kcentra, which is dosed based on INR and body weight. Kcentra contains the vitamin K dependent clotting factors (II, VII, IX, and X) as well as proteins C and S. The patient should also be given vitamin K intravenously to maintain vitamin K-dependent clotting factor levels once the effects of Kcentra have diminished. Kcentra is preferred over a fresh frozen plasma infusion because of its smaller volume, faster infusion rate, and superior effectiveness in INR reduction. For nonmajor bleeding (defined as any bleeding not classified as major) that does not require hospitalization or supportive care, holding vitamin K antagonists and possible administration of vitamin K is recommended depending on INR.
The management of nonmajor bleeding with direct oral anticoagulant therapy typically involves holding the anticoagulant and implementing control measures. Direct oral anticoagulants have half-lives of approximately 12 hours; therefore, holding a dose results in a relatively fast decline in anticoagulant effect. Idarucizumab (Praxbind) is a monoclonal antibody fragment that binds directly to dabigatran, leading to 88% to 98% of patients having concentrations of unbound dabigatran in safe levels within 15 minutes of administration and hemostasis restoration at a median of 11.4 hours.29 For patients taking dabigatran, idarucizumab is recommended for life-threatening or ongoing bleeding, as well as the need to reverse for emergent procedures.
Andexanet alfa (Andexxa) is a genetically modified variant of factor Xa that binds and sequesters factor Xa inhibitors. Andexanet alfa has been approved to reverse the anticoagulant effects of rivaroxaban and apixaban in patients with life-threatening or uncontrolled bleeding. The ANNEXA-4 study showed that 79% of patients with acute major bleeding treated with andexanet alfa achieved effective hemostasis.30 Key selected exclusion criteria from this study included impending surgery, a major thrombotic event within two weeks before enrollment, intracranial hemorrhage with a Glasgow Coma Scale score of less than 7, or receipt of one of the following agents within seven days before screening: vitamin K antagonist, dabigatran, 4-factor prothrombin complex concentrate, blood, or plasma. Of note, 18% of patients had a thrombotic event during 30 days of follow-up, highlighting the potential prothrombotic risk that this agent carries. Optimal dose, duration, and the need for repeat dosing and mitigation of thromboembolic risk have yet to be delineated. Cost can limit availability and use of andexanet alfa.
VTE Treatment and Cancer
VTE treatment in patients with active cancer is challenging because of the increased risk of VTE recurrence and bleeding related to therapy.31–33 Guidelines have recommended LMWH as the anticoagulant of choice for patients with cancer and VTE.1 Evidence is emerging for the increased use of direct oral anticoagulants for certain patients with cancer. The 2019 National Comprehensive Cancer Network guidelines on cancer-associated VTE include rivaroxaban as a monotherapy option.34 Apixaban is included as an acceptable alternative for patients who refuse LMWH or who have compelling reasons to avoid LMWH.35
The SELECT-D trial evaluated dalteparin with rivaroxaban in patients with active cancer.2 The study found an absolute VTE recurrence reduction of 7% at six months in favor of rivaroxaban (11% vs. 4%; hazard ratio [HR] = 0.43; 95% CI, 0.19 to 0.99).2 The major bleeding rate at six months was 4% for dalteparin and 6% for rivaroxaban (HR = 1.83; 95% CI, 0.68 to 4.96).2 Most of the major bleeding was gastrointestinal, primarily in patients with esophageal or gastroesophageal cancer. Clinically relevant nonmajor bleeding was higher in the rivaroxaban group (13% vs. 4%; HR = 3.76; 95% CI, 1.63 to 8.69). The Hokusai VTE cancer trial evaluated dalteparin with edoxaban in patients with active cancer.3 The primary outcome (recurrent VTE and/or major bleeding) did not differ between treatment groups (P = .006 for noninferiority).3 There was a decrease in recurrent deep venous thrombosis in favor of the edoxaban group (3.6% vs. 6.7%; HR = 0.56; CI, 0.32 to 0.97) but an increase in major bleeding in that group (6.9% vs. 4.0%; HR = 1.77; 95% CI, 1.03 to 3.04).3 Gastrointestinal malignancy was also found to be a risk factor of increased gastrointestinal bleeding when using a direct oral anticoagulant vs. LMWH.3 Therefore, direct oral anticoagulants should be used with caution in patients with cancer who have a history of gastrointestinal malignancy or bleeding.
This article updates previous articles on this topic by Wigle, et al.,36 and du Breuil and Umland.37
Data Sources: A PubMed search was completed in Clinical Queries using the key terms outpatient, anticoagulation, warfarin, dabigatran, rivaroxaban, apixaban, edoxaban, betrixaban, heparin, low-molecular-weight heparin, dalteparin, enoxaparin, patient self-monitor, and INR. The search included meta-analyses, randomized controlled trials, clinical trials, clinical guidelines, and reviews. Also searched were the National Guideline Clearinghouse, Essential Evidence Plus, UpToDate, the Cochrane database, and the Agency for Healthcare Research and Quality Evidence Reports. Search dates: June 28, 2018, and May 2, 2019.