
Am Fam Physician. 2019;100(8):505-507
Published online September 20, 2019.
Author disclosure: No relevant financial affiliations.
Key Points for Practice
• Children six months to eight years of age who require two doses of influenza vaccine this season should be vaccinated as soon as possible after vaccine is available to ensure that they receive a second dose by the end of October.
• Early vaccination in people who require only one dose this season is likely to be associated with suboptimal immunity before the end of the influenza season, particularly among older adults.
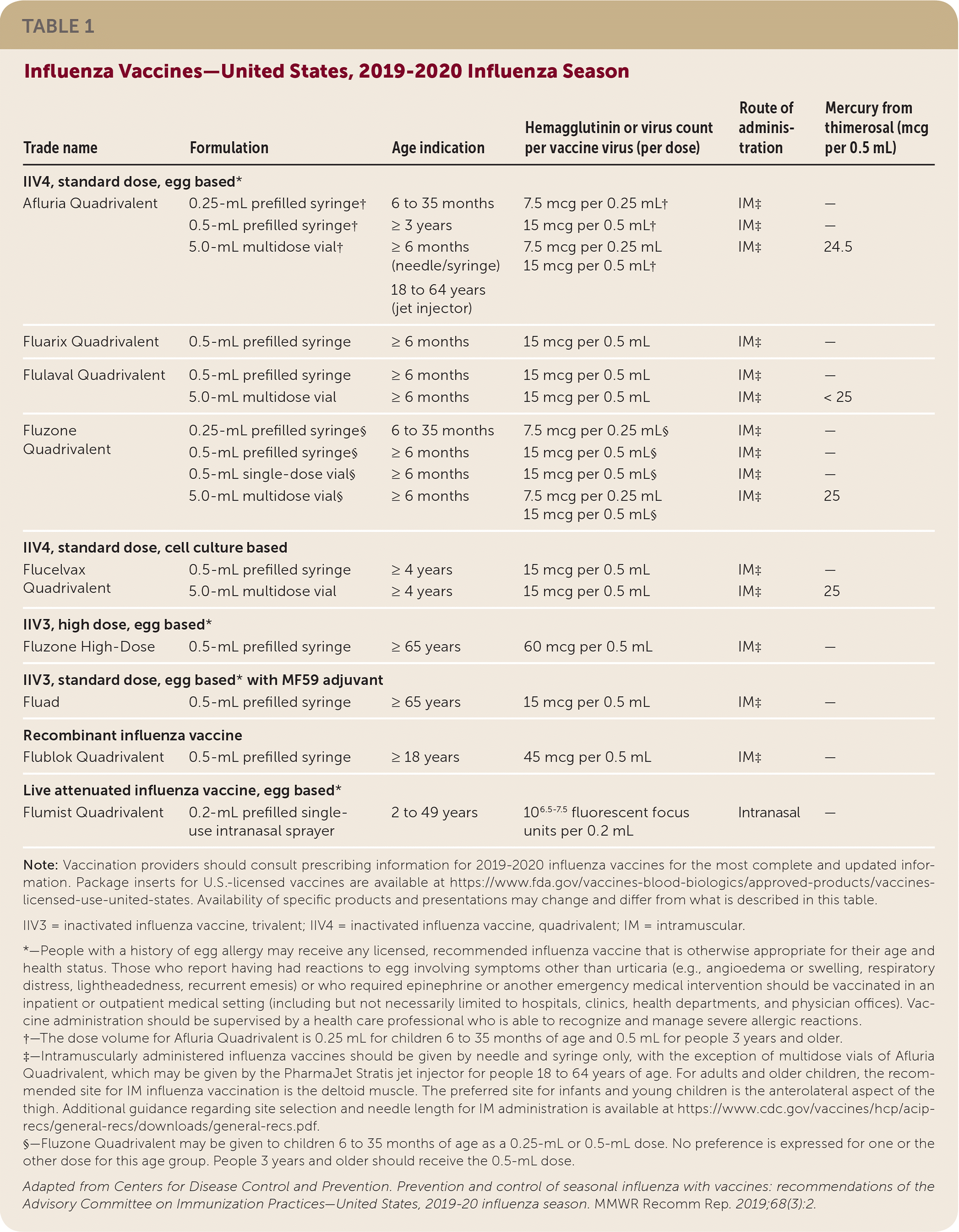
• Afluria Quadrivalent, previously licensed for use in people five years and older, is now licensed for people six months and older.
• Fluzone Quadrivalent can now be given to children six to 35 months of age in either a 0.25-mL or 0.5-mL dose.
From the AFP Editors
The Centers for Disease Control and Prevention's Advisory Committee on Immunization Practices (ACIP) has released its recommendations for routine influenza vaccination in the 2019–2020 season. Updates this year include the antigenic composition of seasonal influenza vaccines available in the United States and changes related to recent regulatory actions, including labeling changes for previously licensed vaccines that occurred after ACIP's vaccination recommendations for the 2018-2019 influenza season were released.
Routine annual influenza vaccination is recommended for all people six months and older who do not have contraindications (see Table 2 at https://www.cdc.gov/mmwr/volumes/68/rr/rr6803a1.htm#T2_down). Contraindications to vaccination include a history of severe allergic reaction, and caution should be used in patients with moderate to severe acute illness or a history of Guillain-Barré syndrome. Vaccination should be offered by the end of October, although vaccine administered in December or later—even if influenza activity has already begun—is likely beneficial in most influenza seasons. Children six months to eight years of age who require two doses of vaccine this season (i.e., those who have not received at least two doses of trivalent or quadrivalent inactivated influenza vaccine before July 2019, or those whose influenza vaccination history is not known) should be vaccinated as soon as possible after vaccine is available so that the second dose can be administered by the end of October. The interval between doses should be at least four weeks. For people who require only one dose this season, early vaccination (July or August) is likely to be associated with suboptimal immunity before the end of the influenza season, particularly among older adults. ACIP did not make a recommendation on whether booster vaccinations should be offered later in the season for people who have already been fully vaccinated. Vaccination should be offered as long as influenza viruses are circulating and unexpired vaccine is available.
Inactivated influenza vaccines, recombinant influenza vaccine, and live attenuated influenza vaccine (LAIV) are expected to be available for the 2019–2020 influenza season (Table 1). Standard-dose, unadjuvanted, inactivated influenza vaccines will be available in quadrivalent formulations. High-dose and adjuvanted inactivated vaccines will be available in trivalent formulations. Recombinant influenza vaccine and LAIV will be available in quadrivalent formulations. For the 2019–2020 influenza season, all vaccines will be egg based, with the exception of Flucelvax Quadrivalent (which is cell culture based) and Flublok Quadrivalent recombinant vaccine. For many vaccine recipients, more than one type or brand may be appropriate; in these cases, no specific vaccine is preferred.
| Trade name | Formulation | Age indication | Hemagglutinin or virus count per vaccine virus (per dose) | Route of administration | Mercury from thimerosal (mcg per 0.5 mL) |
|---|---|---|---|---|---|
| IIV4, standard dose, egg based* | |||||
| Afluria Quadrivalent | 0.25-mL prefilled syringe† | 6 to 35 months | 7.5 mcg per 0.25 mL† | IM‡ | — |
| 0.5-mL prefilled syringe† | ≥ 3 years | 15 mcg per 0.5 mL† | IM‡ | — | |
| 5.0-mL multidose vial† | ≥ 6 months (needle/syringe) | 7.5 mcg per 0.25 mL | IM‡ | 24.5 | |
| 18 to 64 years (jet injector) | 15 mcg per 0.5 mL† | ||||
| Fluarix Quadrivalent | 0.5-mL prefilled syringe | ≥ 6 months | 15 mcg per 0.5 mL | IM‡ | — |
| Flulaval Quadrivalent | 0.5-mL prefilled syringe | ≥ 6 months | 15 mcg per 0.5 mL | IM‡ | — |
| 5.0-mL multidose vial | ≥ 6 months | 15 mcg per 0.5 mL | IM‡ | < 25 | |
| Fluzone Quadrivalent | 0.25-mL prefilled syringe§ | 6 to 35 months | 7.5 mcg per 0.25 mL§ | IM‡ | — |
| 0.5-mL prefilled syringe§ | ≥ 6 months | 15 mcg per 0.5 mL§ | IM‡ | — | |
| 0.5-mL single-dose vial§ | ≥ 6 months | 15 mcg per 0.5 mL§ | IM‡ | — | |
| 5.0-mL multidose vial§ | ≥ 6 months | 7.5 mcg per 0.25 mL | IM‡ | 25 | |
| 15 mcg per 0.5 mL§ | |||||
| IIV4, standard dose, cell culture based | |||||
| Flucelvax | 0.5-mL prefilled syringe | ≥ 4 years | 15 mcg per 0.5 mL | IM‡ | — |
| Quadrivalent | 5.0-mL multidose vial | ≥ 4 years | 15 mcg per 0.5 mL | IM‡ | 25 |
| IIV3, high dose, egg based* | |||||
| Fluzone High-Dose | 0.5-mL prefilled syringe | ≥ 65 years | 60 mcg per 0.5 mL | IM‡ | — |
| IIV3, standard dose, egg based*with MF59 adjuvant | |||||
| Fluad | 0.5-mL prefilled syringe | ≥ 65 years | 15 mcg per 0.5 mL | IM‡ | — |
| Recombinant influenza vaccine | |||||
| Flublok Quadrivalent | 0.5-mL prefilled syringe | ≥ 18 years | 45 mcg per 0.5 mL | IM‡ | — |
| Live attenuated influenza vaccine, egg based* | |||||
| Flumist Quadrivalent | 0.2-mL prefilled single-use intranasal sprayer | 2 to 49 years | 106.5–7.5 fluorescent focus units per 0.2 mL | Intranasal | — |
Trivalent influenza vaccines available for the 2019–2020 influenza season will contain hemagglutinin derived from an A/Brisbane/02/2018 (H1N1)pdm09-like virus, an A/Kansas/14/2017(H3N2)-like virus, and a B/Colorado/06/2017-like virus (Victoria lineage). Quadrivalent vaccines will contain hemagglutinin derived from these three viruses and an additional virus: a B/Phuket/3073/2013-like virus (Yamagata lineage). The trivalent and quadrivalent vaccines contain different influenza A (H3N2) and influenza A (H1N1)pdm09 strains compared with the 2018–2019 influenza vaccines.
In October 2018, the U.S. Food and Drug Administration (FDA) approved an expanded age indication for Afluria Quadrivalent, which was previously licensed for use in people five years and older. The vaccine is now licensed for all people six months and older. Children six to 35 months of age should receive a 0.25-mL dose, which contains 7.5 mcg of hemagglutinin per vaccine virus; all other recipients should receive a 0.5-mL dose, which contains 15 mcg of hemagglutinin per virus.
In January 2019, the FDA approved a change in dose volume for Fluzone Quadrivalent. Children six to 35 months of age can now receive either a 0.25-mL dose, which contains 7.5 mcg of hemagglutinin per vaccine virus, or a 0.5-mL dose, which contains 15 mcg of hemagglutinin per virus. Children three years and older and adults should receive a 0.5-mL dose.
People of all ages are susceptible to seasonal influenza, but vaccination is particularly important in people at increased risk of severe complications or influenza-related hospitalizations, as well as those who live with or care for such people. When vaccine supply is limited, vaccination efforts should target the following groups:
Adults 50 years and older
American Indians/Alaska Natives
Children and adolescents six months to 18 years of age who are receiving aspirin- or salicylate-containing medications and may be at risk of Reye syndrome after influenza virus infection
Children six to 59 months of age
Health care professionals and students, and people not directly involved in patient care but who may be exposed to infectious agents
Household contacts and caregivers of children younger than five years, adults 50 years or older, or people with medical conditions that increase their risk of severe influenza-related complications
Immunocompromised people
People with a body mass index of 40 kg per m2 or greater
People with chronic pulmonary, cardiovascular (excluding isolated hypertension), renal, hepatic, neurologic, hematologic, or metabolic disorders (e.g., asthma, diabetes mellitus)
Pregnant women
Residents of nursing homes or other long-term care facilities
Editor's Note: Recommendations on the use of LAIV from both the American Academy of Family Physicians and the American Academy of Pediatrics are consistent with the ACIP recommendations (https://pediatrics.aappublications.org/content/early/2019/08/29/peds.2019-2478 and https://www.aafp.org/patient-care/public-health/immunizations/influenza.html). In the 2018–2019 influenza season, both groups preferred inactivated vaccine over LAIV. This year there is no preference. — Sumi M. Sexton, MD, Editor-in-Chief
Guideline source: Advisory Committee on Immunization Practices
Evidence rating system used? No
Systematic literature search described? No
Guideline developed by participants without relevant financial ties to industry? Yes
Recommendations based on patient-oriented outcomes? Yes
Published source: MMWR Recomm Rep. August 23, 2019;68(3):1–21
Available at: https://www.cdc.gov/mmwr/volumes/68/rr/rr6803a1.htm
