Am Fam Physician. 2019;100(11):672-675
Author disclosure: No relevant financial affiliations.
Gabapentinoid drugs—specifically gabapentin (Neurontin) and pregabalin (Lyrica)—are increasingly being prescribed for pain because physicians and patients seek alternatives to opioids in the midst of the opioid crisis.1,2 However, such widespread and often indiscriminate prescribing of gabapentinoids is not supported by robust evidence, and it carries known and unknown risks. Gabapentin was first approved by the U.S. Food and Drug Administration (FDA) for the treatment of seizures in 1993 and was subsequently approved for one pain indication, postherpetic neuralgia. Pregabalin was first marketed in 2004 and is currently FDA approved for the pain indications of diabetic neuropathy, postherpetic neuralgia, fibromyalgia, and pain associated with spinal cord injury. Despite the small number of indications, an estimated 4% of U.S. adults were prescribed gabapentinoids at least once in 2015.2
The transition of gabapentinoids into a first-line pain medication is in part due to an intentional marketing strategy by the pharmaceutical industry (now well documented in the medical literature) that involved widely promoting off-label use with low-quality, industry-funded studies manipulated to exaggerate the perceived analgesic effects of these drugs.3–5 In our recently published review of randomized placebo-controlled trials of gabapentinoids for noncancer pain conditions outside of FDA-approved indications, most results were either negative or not clinically significant (Table 1).6 The idea that these drugs are highly effective first-line options for any pain defined as neuropathic is simply incorrect. Guidelines and review articles do physicians and patients a disservice when they extrapolate benefits from trials conducted primarily in patients with postherpetic neuralgia and painful diabetic neuropathy to patients with other types of neuropathic pain.
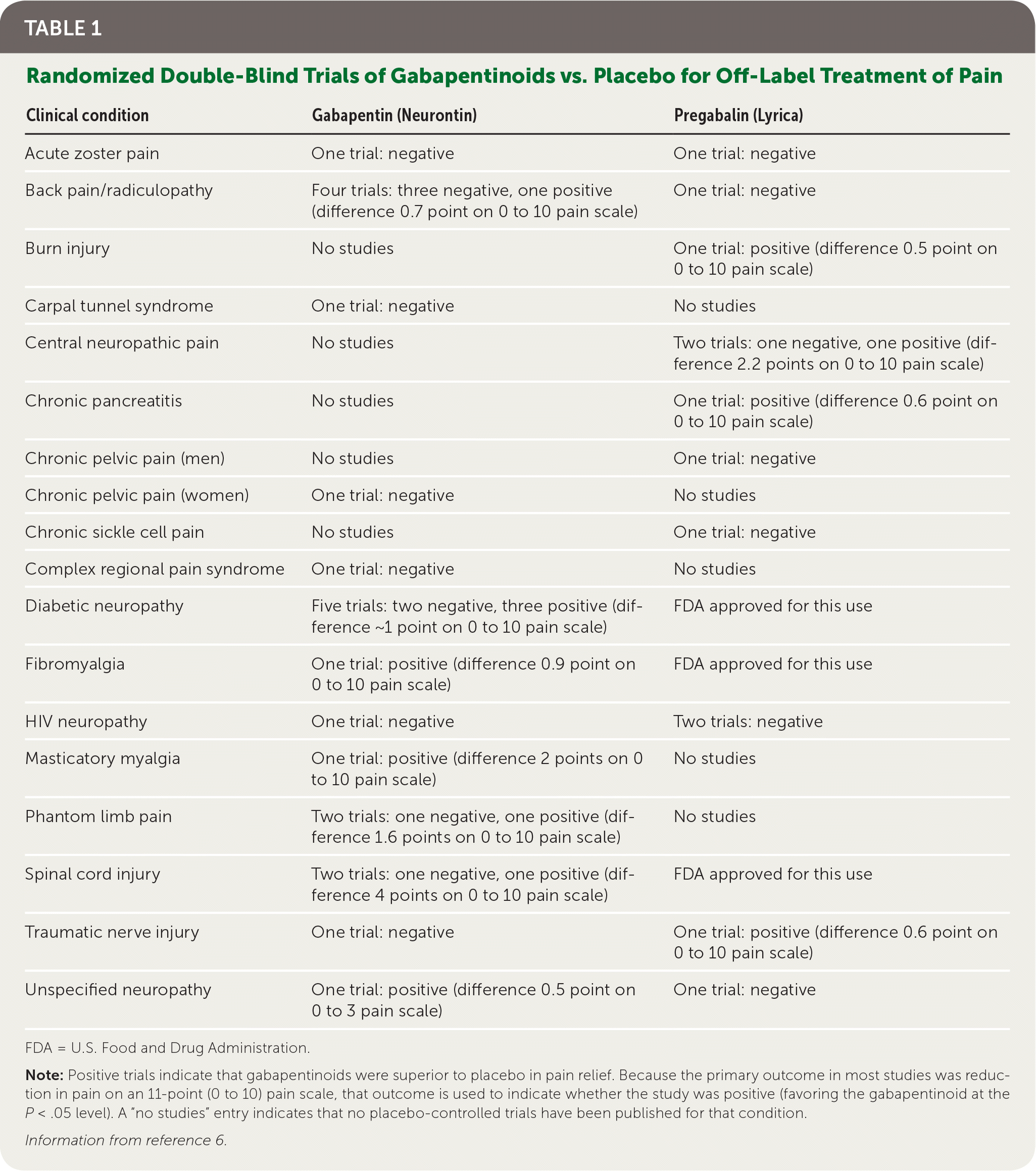
| Clinical condition | Gabapentin (Neurontin) | Pregabalin (Lyrica) |
|---|---|---|
| Acute zoster pain | One trial: negative | One trial: negative |
| Back pain/radiculopathy | Four trials: three negative, one positive (difference 0.7 point on 0 to 10 pain scale) | One trial: negative |
| Burn injury | No studies | One trial: positive (difference 0.5 point on 0 to 10 pain scale) |
| Carpal tunnel syndrome | One trial: negative | No studies |
| Central neuropathic pain | No studies | Two trials: one negative, one positive (difference 2.2 points on 0 to 10 pain scale) |
| Chronic pancreatitis | No studies | One trial: positive (difference 0.6 point on 0 to 10 pain scale) |
| Chronic pelvic pain (men) | No studies | One trial: negative |
| Chronic pelvic pain (women) | One trial: negative | No studies |
| Chronic sickle cell pain | No studies | One trial: negative |
| Complex regional pain syndrome | One trial: negative | No studies |
| Diabetic neuropathy | Five trials: two negative, three positive (difference ~1 point on 0 to 10 pain scale) | FDA approved for this use |
| Fibromyalgia | One trial: positive (difference 0.9 point on 0 to 10 pain scale) | FDA approved for this use |
| HIV neuropathy | One trial: negative | Two trials: negative |
| Masticatory myalgia | One trial: positive (difference 2 points on 0 to 10 pain scale) | No studies |
| Phantom limb pain | Two trials: one negative, one positive (difference 1.6 points on 0 to 10 pain scale) | No studies |
| Spinal cord injury | Two trials: one negative, one positive (difference 4 points on 0 to 10 pain scale) | FDA approved for this use |
| Traumatic nerve injury | One trial: negative | One trial: positive (difference 0.6 point on 0 to 10 pain scale) |
| Unspecified neuropathy | One trial: positive (difference 0.5 point on 0 to 3 pain scale) | One trial: negative |
Gabapentinoids have significant risks despite their reputation as safe drugs. Central nervous system effects such as sedation, dizziness, gait instability, and feeling intoxicated are quite common; as many as one in three patients taking therapeutic doses will experience dizziness or somnolence.7,8 Additionally, in our experience with caring for hospitalized patients and general medical outpatients, these drugs are being prescribed liberally to older adults or patients with multiple comorbidities who are at risk for polypharmacy. In such patients, adverse effects of gabapentinoids tend to be underrecognized, and the requirement for substantially lower doses with renal impairment is frequently overlooked.
Evidence regarding misuse and diversion of gabapentinoids has been growing. Current research suggests that the addictive potential of gabapentinoids is primarily a concern among patients with other substance use disorders, especially opioid use disorder.9 The higher prevalence of gabapentinoid misuse among patients with opioid use disorder compared to those with other substance use is thought to relate to augmentation of euphoria. Concomitant use of opioids and gabapentinoids is associated with an increased risk of hospitalization (compared with gabapentinoid monotherapy) and opioid-related death (compared with opioid monotherapy); the interaction between opioids and gabapentinoids has led to an update of the Beers criteria cautioning against dual therapy in older adults.10–12 Several states have moved to make gabapentinoids controlled substances for closer monitoring, and the FDA has publicly noted an intention to consider a federal change in regulation.13
The opioid crisis has likely resulted in undertreatment of pain when physicians have substituted other drugs (including gabapentinoids) that might be less effective in a given case. Because physicians are feeling intense pressure to avoid opioid prescribing, they may be withholding opioids from patients who have used—or will use—modest doses responsibly and effectively.14 The authors of the frequently referenced 2016 guideline regarding opioid prescribing from the Centers for Disease Control and Prevention recently published a statement acknowledging that some recent policies and practices have been inconsistent with, and often go beyond, the Centers for Disease Control and Prevention recommendations.15,16 Whereas we do not advocate using opioids as first-line treatment for chronic noncancer pain, opioid prescribing may be beneficial in carefully selected cases if physicians adhere to treatment guidelines.
Management of patients with chronic pain in primary care practice can be difficult. When faced with patients who are struggling with pain, the path of least resistance is often to write a prescription and move on, particularly during brief office visits. Instead of relying on medications, physicians and patients should prioritize the use of nonpharmacologic treatment options, including mindfulness, behavioral therapy, movement-based therapies, exercise, and physical therapy, which have less potential for harm and may confer other health benefits.17
Although accessibility and affordability are concerns for certain nonpharmacologic treatments, informal resources for some treatments are extensive, especially considering the potential for self-directed treatment (e.g., yoga, tai chi, mindfulness, exercise).
We occasionally offer patients gabapentinoids off-label; during those clinical encounters, we note to patients that the supporting evidence is limited, review potential adverse effects, and agree on a limited time frame during which the net effect of the drug will be evaluated. If the patient does not clearly experience benefit, the drug should be discontinued. Because gabapentinoids have potential for withdrawal syndrome, they should be tapered gradually over a minimum of one week to minimal dosages (e.g., 300 mg daily for gabapentin, 75 mg daily for pregabalin) before they are stopped.