
Am Fam Physician. 2019;100(12):759-770
Author disclosure: No relevant financial affiliations.
Cirrhosis is the 12th leading cause of death in the United States. Newer research has established that liver fibrosis is a dynamic process and that early cirrhosis may be reversible. Only one in three people with cirrhosis knows they have it. Most patients with cirrhosis remain asymptomatic until the onset of decompensation. When clinical signs, symptoms, or abnormal liver function tests are discovered, further evaluation should be pursued promptly. The most common causes of cirrhosis are viral hepatitis, alcoholic liver disease, and nonalcoholic steatohepatitis. Initial workup includes viral hepatitis serologies, ferritin, transferrin saturation, and abdominal ultrasonography as well as complete blood count, liver function tests, and prothrombin time/international normalized ratio, if not already ordered. Additional testing is based on demographics and risk factors. Common serum and ultrasound-based screening tests to assess fibrosis include the aspartate transaminase to platelet ratio index score, Fibrosis 4 score, FibroTest/FibroSure, nonalcoholic fatty liver fibrosis score, standard ultrasonography, and transient elastography. Generally, noninvasive tests are most useful in identifying patients with no to minimal fibrosis or advanced fibrosis. Chronic liver disease management includes directed counseling, laboratory testing, and ultrasound monitoring. Treatment goals are preventing cirrhosis, decompensation, and death. Varices are monitored with endoscopy and often require prophylaxis with nonselective beta blockers. Ascites treatment includes diuresis, salt restriction, and antibiotic prophylaxis for spontaneous bacterial peritonitis, when indicated. Hepatic encephalopathy is managed with lifestyle and nutritional modifications and, as needed, with lactulose and rifaximin. Hepatocellular carcinoma screening includes ultrasound screening every six months for patients with cirrhosis.
Cirrhosis is a diffuse process of liver damage considered irreversible in its advanced stages. In 2016, more than 40,000 Americans died because of complications related to cirrhosis, making it the 12th leading cause of death in the United States.1 Recent projections suggest that this number is likely to grow.2 An estimated 630,000 Americans have cirrhosis, yet less than one in three knows it.3 Important racial and socioeconomic disparities exist, with prevalence highest among non-Hispanic blacks, Mexican Americans, and those living below the poverty level.3 Cirrhosis and advanced liver disease cost the United States between $12 billion and $23 billion dollars in health care expenses annually.4,5
WHAT'S NEW ON THIS TOPIC
Cirrhosis
Estimates suggest that nonalcoholic steatohepatitis will become the leading cause of cirrhosis in U.S. patients awaiting liver transplantation sometime between 2025 and 2035.
Liver biopsy remains the reference standard; however, transient elastography has become more widely available and is rapidly replacing biopsy as the preferred method for liver fibrosis staging.
Newer guidelines suggest targeted screening for esophageal varices in patients with clinically significant portal hypertension rather than screening all patients with cirrhosis.
The most common causes of cirrhosis in the United States are viral hepatitis (primarily hepatitis C virus [HCV] and hepatitis B virus [HBV]), alcoholic liver disease, and non-alcoholic steatohepatitis. HCV remains the leading cause of cirrhosis in patients awaiting liver transplant. With an increasing prevalence of nonalcoholic fatty liver disease (NAFLD) in the United States, estimates suggest that non-alcoholic steatohepatitis, a severe progression of NAFLD characterized by inflammatory steatohepatitis, will become the leading cause of cirrhosis in patients awaiting liver transplant sometime between 2025 and 2035.6,7 Table 1 lists common etiologies of cirrhosis.8

| Viral hepatitis (hepatitis B, hepatitis C) |
| Alcoholic liver disease |
| Nonalcoholic fatty liver disease/nonalcoholic steatohepatitis |
| Storage diseases |
| Hemochromatosis |
| Wilson disease |
| Alpha1-antitrypsin deficiency |
| Immune mediated |
| Autoimmune hepatitis (types 1, 2, and 3) |
| Primary biliary cholangitis |
| Primary sclerosing cholangitis |
| Immunoglobulin G4 cholangiopathy |
| Cardiovascular |
| Veno-occlusive disease (Budd-Chiari syndrome) |
| Congestive heart failure |
| Hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu disease) |
| Chronic biliary disease |
| Recurrent bacterial cholangitis |
| Bile duct stenosis |
| Other |
| Medications (e.g., methotrexate, amiodarone) |
| Erythropoietic protoporphyria |
| Sarcoidosis |
| Schistosomiasis |
Pathophysiology and Natural History of Cirrhosis
Chronic liver injury causes inflammation and hepatic fibrosis. Regardless of the cause, this can lead to the formation of fibrous septae and nodules, collapse of liver structures, and distortion of hepatic parenchyma and vascular architecture. Progressive fibrosis and cirrhosis subsequently result in decreased metabolic and synthetic hepatic function, causing a rise in bilirubin and decreased production of clotting factors and thrombopoietin, as well as splenic platelet sequestration, increased portal pressure, and the development of ascites and esophageal varices.
Cirrhosis can result from chronic liver damage of any cause. In patients with the three most common causes of liver disease, 10% to 20% will develop cirrhosis within 10 to 20 years.9 Factors associated with an increased risk of progression to cirrhosis include increased age, medical comorbidities (particularly patients coinfected with HIV and HCV), and male sex (except in alcoholic liver disease, where females progress more rapidly).10 The point at which this process becomes irreversible, however, is not clear. Newer research has established that liver fibrosis is a dynamic process and that even early cirrhosis is reversible.11 Studies have demonstrated biopsy-proven fibrosis improvement rates as high as 88% after antiviral treatment in patients with HBV and HCV and as high as 85% after bariatric surgery in patients with nonalcoholic steatohepatitis.12,13
After cirrhosis is established, a patient may remain clinically stable, or compensated, for years. Patients with compensated cirrhosis caused by HBV, HCV, and alcoholic liver disease develop clinical signs of decompensation, which include ascites, hepatic encephalopathy, jaundice, or bleeding, at a rate of 4% to 10% per year.14 Variability of disease progression is influenced by the underlying cause and the presence or absence of treatment and ongoing liver injury. The median survival for those with compensated cirrhosis is 12 years, compared with two years once decompensation occurs.15
Clinical Presentation
HISTORY
Most patients with compensated cirrhosis remain asymptomatic. When symptoms occur, they include fatigue, weakness, loss of appetite, right upper quadrant discomfort, and unexplained weight loss. With the onset of decompensation, patients may report symptoms of impaired liver function such as jaundice, portal hypertension (including ascites and peripheral edema), and hepatic encephalopathy (such as confusion and disordered sleep).
PHYSICAL EXAMINATION
Physical examination findings that may be present in patients with advanced liver disease (cirrhosis) are summarized in Table 2.16,17 The Stanford Medicine 25 website is a good resource for photos and instructional videos that demonstrate findings associated with cirrhosis (http://stanfordmedicine25.stanford.edu/the25/liverdisease.html).16,17

| Body part/system | Examination finding |
|---|---|
| General | Muscle wasting |
| Central nervous system | Asterixis (tremor of the hand with wrist extension) |
| Drowsiness, confusion | |
| Head | Fetor hepaticus: sweet odor of the breath attributable to increased concentrations of dimethyl sulfide |
| Jaundice: may see yellowing of mucous membranes beneath the tongue | |
| Parotid enlargement | |
| Scleral icterus | |
| Spider nevi | |
| Chest | Gynecomastia |
| Spider nevi | |
| Thinning axillary hair | |
| Abdomen | Ascites |
| Caput medusae (engorged superficial epigastric veins radiating from the umbilicus) | |
| Contracted or enlarged liver | |
| Hemorrhoids | |
| Splenomegaly | |
| Hands and nails | Clubbing |
| Dupuytren contracture (progressive fibrosis of palmar fascia, resulting in limited extension of the fingers) | |
| Palmar erythema | |
| Terry nails (whiteness of proximal half of nail plate) | |
| Genitourinary (male) | Testicular atrophy |
| Lower extremities | Distal erythema |
| Edema | |
| Petechiae |
INITIAL LABORATORY FINDINGS
In early compensated disease, laboratory findings may be normal. Incidentally elevated liver enzymes or evidence of hepatic disease on imaging may prompt the initial suspicion of chronic liver injury. Findings suggestive of cirrhosis include low albumin (less than 3.5 g per dL [35 g per L]), thrombocytopenia (platelet count less than 160 × 103 per μL [160 × 109 per L]), aspartate transaminase (AST):alanine transaminase (ALT) ratio greater than 1, elevated bilirubin, and a prolonged prothrombin time (PT)/elevated international normalized ratio (INR).18
Evaluation of Chronic Liver Disease
When chronic liver disease is suspected, a history should be conducted, reviewing any potentially hepatotoxic medications, alcohol consumption, and family history of liver disease. Basic laboratory tests, including complete blood count, ALT, AST, albumin, alkaline phosphatase, gamma-glutamyl transferase, total bilirubin, and PT/INR, should be ordered.
For those with clinical signs or symptoms of liver disease or abnormal liver function test results, regardless of duration, further evaluation to determine the potential etiology should be pursued promptly.19,20 Viral hepatitis serologies, ferritin, transferrin saturation, and abdominal ultrasonography should be performed; complete blood count, liver function tests, and PT/INR should be completed, if not already ordered. If risk factors for NAFLD exist, testing of fasting lipid levels and A1C should be done. For patients with risk factors or demographics with concern for autoimmune hepatitis, antinuclear antibodies and smooth muscle antibodies should be tested. Table 3 lists additional suggested tests based on risk factors and clinical findings.19,21,22
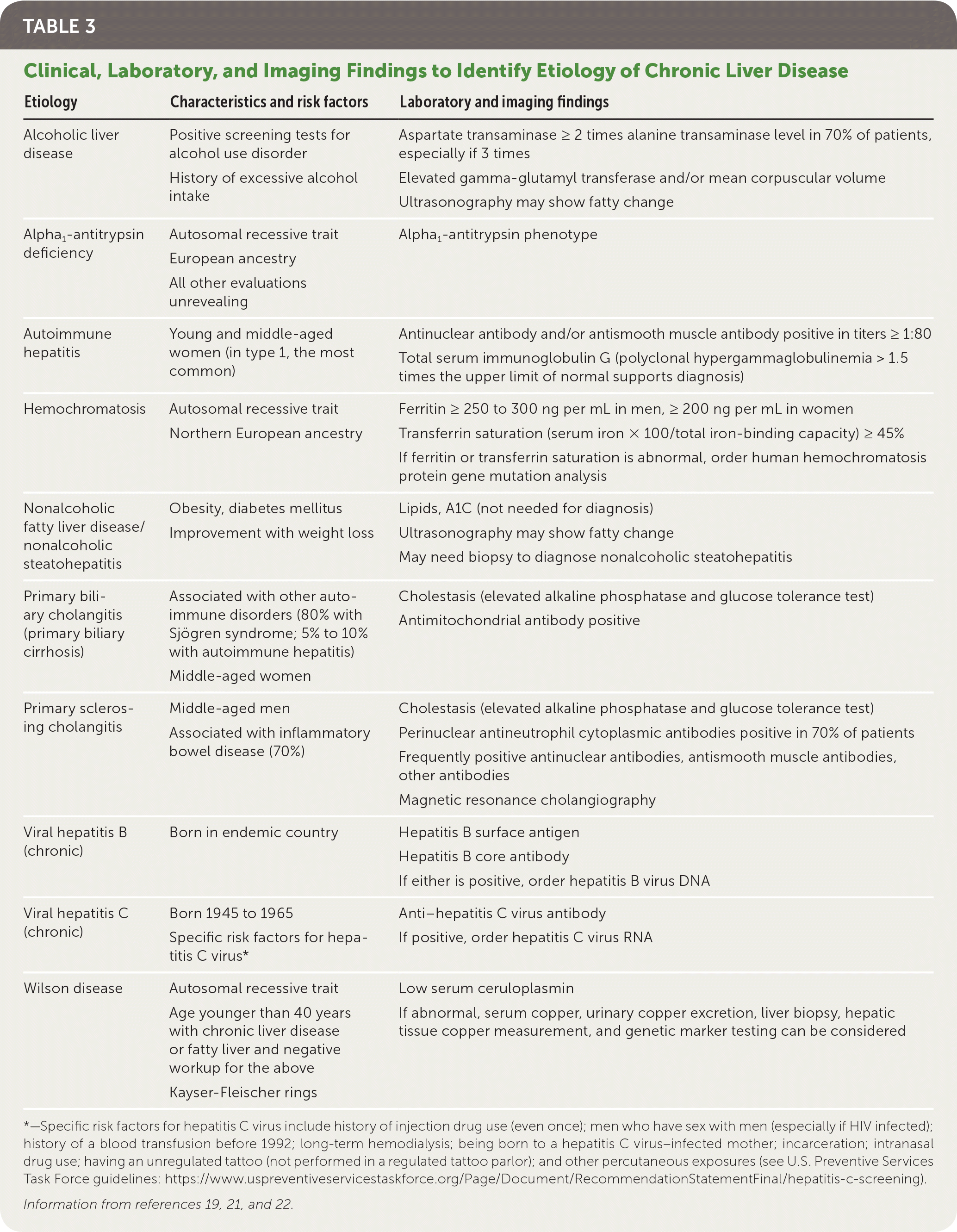
| Etiology | Characteristics and risk factors | Laboratory and imaging findings |
|---|---|---|
| Alcoholic liver disease | Positive screening tests for alcohol use disorder | Aspartate transaminase ≥ 2 times alanine transaminase level in 70% of patients, especially if 3 times |
| History of excessive alcohol intake | Elevated glucose tolerance test and/or mean corpuscular volume [corrected] | |
| Ultrasonography may show fatty change | ||
| Alpha1-antitrypsin deficiency | Autosomal recessive trait | Alpha1-antitrypsin phenotype |
| European ancestry | ||
| All other evaluations unrevealing | ||
| Autoimmune hepatitis | Young and middle-aged women (in type 1, the most common) | Antinuclear antibody and/or antismooth muscle antibody positive in titers ≥ 1:80 |
| Total serum immunoglobulin G (polyclonal hypergammaglobulinemia > 1.5 times the upper limit of normal supports diagnosis) | ||
| Hemochromatosis | Autosomal recessive trait | Ferritin ≥ 250 to 300 ng per mL in men, ≥ 200 ng per mL in women |
| Northern European ancestry | Transferrin saturation (serum iron × 100/total iron-binding capacity) ≥ 45% | |
| If ferritin or transferrin saturation is abnormal, order human hemochromatosis protein gene mutation analysis | ||
| Nonalcoholic fatty liver disease/nonalcoholic steatohepatitis | Obesity, diabetes mellitus | Lipids, A1C (not needed for diagnosis) |
| Improvement with weight loss | Ultrasonography may show fatty change | |
| May need biopsy to diagnose nonalcoholic steatohepatitis | ||
| Primary biliary cholangitis (primary biliary cirrhosis) | Associated with other autoimmune disorders (80% with Sjögren syndrome; 5% to 10% with autoimmune hepatitis) Middle-aged women | Cholestasis (elevated alkaline phosphatase and glucose tolerance test) Antimitochondrial antibody positive |
| Primary sclerosing cholangitis | Middle-aged men | Cholestasis (elevated alkaline phosphatase and glucose tolerance test) |
| Associated with inflammatory bowel disease (70%) | Perinuclear antineutrophil cytoplasmic antibodies positive in 70% of patients Frequently positive antinuclear antibodies, antismooth muscle antibodies, other antibodies Magnetic resonance cholangiography | |
| Viral hepatitis B (chronic) | Born in endemic country | Hepatitis B surface antigen |
| Hepatitis B core antibody | ||
| If either is positive, order hepatitis B virus DNA | ||
| Viral hepatitis C (chronic) | Born 1945 to 1965 | Anti–hepatitis C virus antibody |
| Specific risk factors for hepatitis C virus* | If positive, order hepatitis C virus RNA | |
| Wilson disease | Autosomal recessive trait | Low serum ceruloplasmin |
| Age younger than 40 years with chronic liver disease or fatty liver and negative workup for the above | If abnormal, serum copper, urinary copper excretion, liver biopsy, hepatic tissue copper measurement, and genetic marker testing can be considered | |
| Kayser-Fleischer rings |
Staging Fibrosis and Diagnosing Cirrhosis
Liver fibrosis is scored on a scale from F0 to F4 (Table 4).23 Differentiating between significant (F2 or greater) and advanced (F3 or greater) fibrosis and cirrhosis (F4) is difficult even with complete clinical, laboratory, and imaging data because findings are often nonspecific or insensitive.24 Liver biopsy remains the reference standard for assessing liver fibrosis; however, use of noninvasive methods has become increasingly common in clinical practice.18
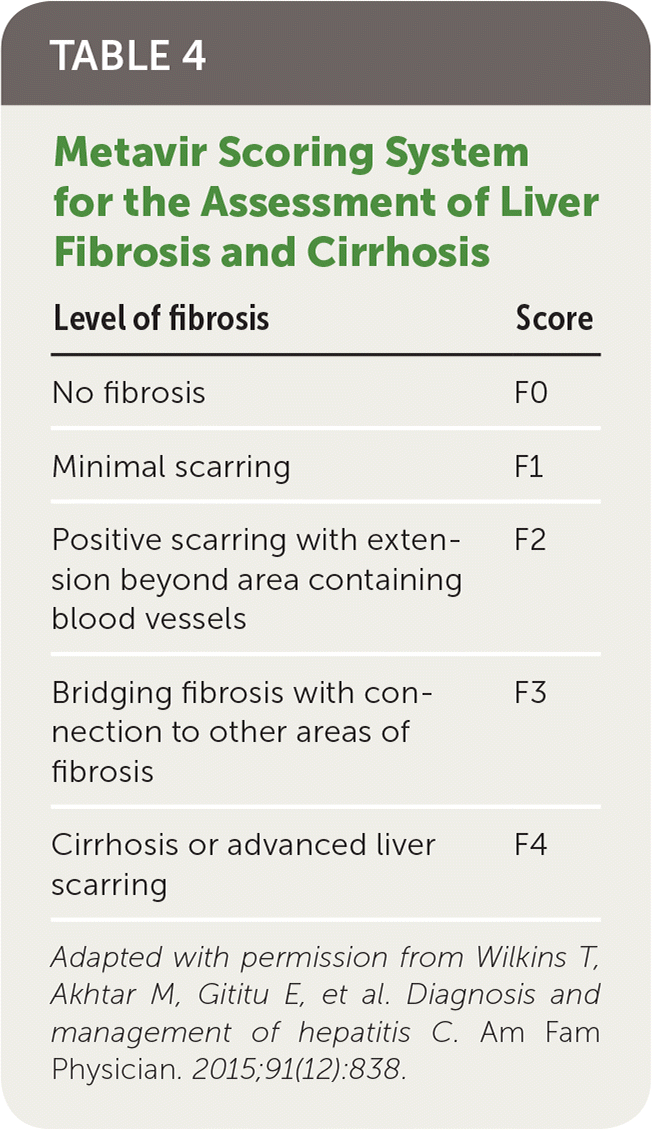
| Level of fibrosis | Score |
|---|---|
| No fibrosis | F0 |
| Minimal scarring | F1 |
| Positive scarring with extension beyond area containing blood vessels | F2 |
| Bridging fibrosis with connection to other areas of fibrosis | F3 |
| Cirrhosis or advanced liver scarring | F4 |
Noninvasive testing includes serum-based and imaging modalities (Table 525–37). Generally, noninvasive tests are most useful in identifying patients with no to minimal fibrosis (F0) or advanced fibrosis (F3 to F4) and are less accurate at distinguishing early or intermediate stages of liver disease (F1 to F2).24,38 They are most beneficial when combined with all available data, accounting for the pretest probability of fibrosis.24,38
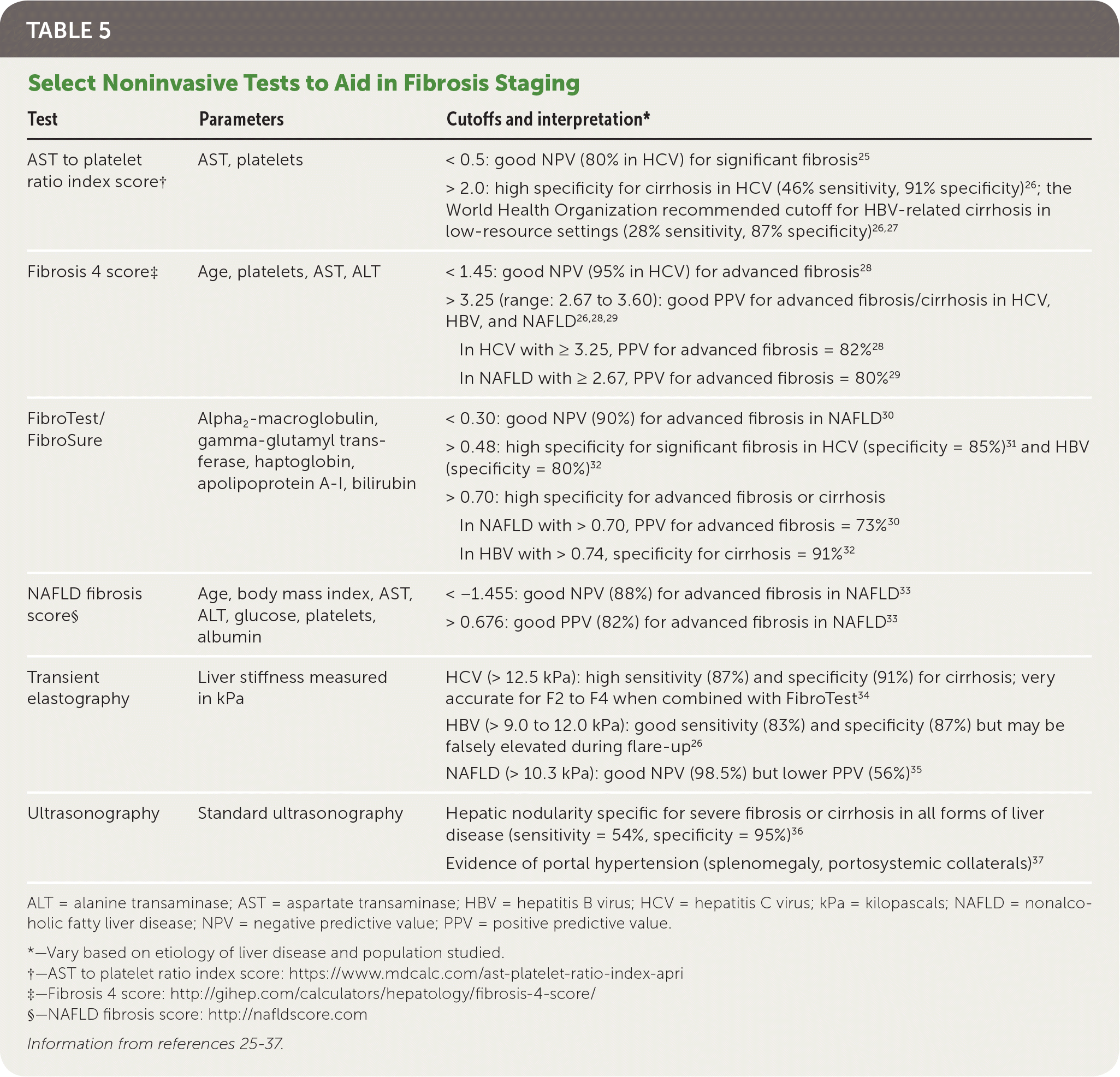
| Test | Parameters | Cutoffs and interpretation* |
|---|---|---|
| AST to platelet ratio index score† | AST, platelets | < 0.5: good NPV (80% in HCV) for significant fibrosis25 |
| > 2.0: high specificity for cirrhosis in HCV (46% sensitivity, 91% specificity)26; the World Health Organization recommended cutoff for HBV-related cirrhosis in low-resource settings (28% sensitivity, 87% specificity)26,27 | ||
| Fibrosis 4 score‡ | Age, platelets, AST, ALT | < 1.45: good NPV (95% in HCV) for advanced fibrosis28 |
| > 3.25 (range: 2.67 to 3.60): good PPV for advanced fibrosis/cirrhosis in HCV, HBV, and NAFLD26,28,29 | ||
| In HCV with ≥ 3.25, PPV for advanced fibrosis = 82%28 | ||
| In NAFLD with ≥ 2.67, PPV for advanced fibrosis = 80%29 | ||
| FibroTest/FibroSure | Alpha2 -macroglobulin, gamma-glutamyl transferase, haptoglobin, apolipoprotein A-I, bilirubin | < 0.30: good NPV (90%) for advanced fibrosis in NAFLD30 |
| > 0.48: high specificity for significant fibrosis in HCV (specificity = 85%)31 and HBV (specificity = 80%)32 | ||
| > 0.70: high specificity for advanced fibrosis or cirrhosis | ||
| In NAFLD with > 0.70, PPV for advanced fibrosis = 73%30 | ||
| In HBV with > 0.74, specificity for cirrhosis = 91%32 | ||
| NAFLD fibrosis score§ | Age, body mass index, AST, ALT, glucose, platelets, albumin | < −1.455: good NPV (88%) for advanced fibrosis in NAFLD33 |
| > 0.676: good PPV (82%) for advanced fibrosis in NAFLD33 | ||
| Transient elastography | Liver stiffness measured in kPa | HCV (> 12.5 kPa): high sensitivity (87%) and specificity (91%) for cirrhosis; very accurate for F2 to F4 when combined with FibroTest34 |
| HBV (> 9.0 to 12.0 kPa): good sensitivity (83%) and specificity (87%) but may be falsely elevated during flare-up26 | ||
| NAFLD (> 10.3 kPa): good NPV (98.5%) but lower PPV (56%)35 | ||
| Ultrasonography | Standard ultrasonography | Hepatic nodularity specific for severe fibrosis or cirrhosis in all forms of liver disease (sensitivity = 54%, specificity = 95%)36 |
| Evidence of portal hypertension (splenomegaly, portosystemic collaterals)37 |
BIOMARKERS
Most serum tests show indirect markers of liver damage, except hyaluronic acid (found in the liver's extracellular matrix), which is included in biomarker panels such as FibroMeter or Hepascore.24 The AST to platelet ratio index (APRI; https://www.mdcalc.com/ast-platelet-ratio-index-apri), Fibrosis 4 score (http://gihep.com/calculators/hepatology/fibrosis-4-score/), and NAFLD fibrosis score (http://nafldscore.com/) are accessible, serum-based, nonproprietary calculations.18,39 FibroTest (FibroSure in the United States), FibroMeter, and Hepascore are patented calculations using several serum biomarkers, with FibroTest being the most validated.24
STANDARD ULTRASONOGRAPHY
Given its relatively low cost, accessibility, and lack of radiation, ultrasonography is useful for diagnosing cirrhosis, cirrhosis complications (e.g., splenomegaly, portal hypertension, ascites, hepatocellular carcinoma), and comorbid liver diseases (e.g., extrahepatic cholestasis).24 Ultrasonography is good at detecting steatosis (94% sensitivity, 84% specificity), but it may frequently miss fibrosis or cirrhosis (for which it is 40% to 57% sensitive).41,42 Characteristics of cirrhosis include hepatic nodularity, coarseness, and echogenicity,24 with hepatic nodularity being the most specific.36 Additionally, features consistent with portal hypertension, such as splenomegaly and portosystemic collaterals, are suggestive of cirrhosis.37 Patients with cirrhosis and some with chronic HBV should undergo right upper quadrant ultrasonography every six months to screen for hepatocellular carcinoma.43
TRANSIENT ELASTOGRAPHY
Transient elastography, which has become more widely available, is rapidly replacing biopsy as the preferred method for fibrosis staging. Transient elastography, an ultrasound technique performed with a specialized machine (Fibro-Scan), determines liver stiffness in kilopascals (kPa) by measuring the velocity of low-frequency elastic shear waves propagating through the liver. It is a five-minute procedure performed in an outpatient setting and provides point-of-care results. In a meta-analysis of more than 10,000 patients spanning multiple etiologies of liver disease, transient elastography was sensitive (81%) and specific (88%) for detecting liver fibrosis and cirrhosis40 (see Table 525–37 for cutoff values). Transient elastography performs better than the biomarker-based tools in detecting cirrhosis and is accurate at excluding cirrhosis (negative predictive value greater than 90%).38 Similar to serum tests, however, transient elastography is less accurate at distinguishing between intermediate stages of liver disease, and cutoff values vary depending on the etiology of liver disease and population studied.24,38
LIMITATIONS
Abnormal serum results may be seen from non–liver-related causes, including bone marrow disease, hemolysis, and medications. Transient elastography is less reliable in patients with obesity (though an extra-large probe has been developed), ascites, excessive alcohol intake, and extrahepatic cholestasis. If performed during an episode of acute hepatic inflammation, these tests can also lead to falsely elevated results.38
LIVER BIOPSY
Liver biopsy remains the reference standard in diagnosing cirrhosis; however, a 20% error rate still occurs in fibrosis staging.44 Pathologic changes may be heterogeneous; therefore, sampling error is common, and interpretation should be made by an experienced pathologist using validated scoring systems.38 Liver biopsy is recommended when concern for fibrosis remains after indeterminate or conflicting clinical, laboratory, and imaging results; in those for whom transient elastography is not suitable; or to clarify etiology of disease after inconclusive noninvasive evaluation.9 Liver biopsy may be indicated to diagnose necroinflammation (in HBV) and steatohepatitis (nonalcoholic steatohepatitis) because they are not easily distinguished by noninvasive methods.
Staging Cirrhosis
After the diagnosis of cirrhosis is established, Child-Pugh (https://www.mdcalc.com/child-pugh-score-cirrhosis-mortality) and Model for End-Stage Liver Disease (https://www.mdcalc.com/meld-score-model-end-stage-liver-disease-12-older) scores should be used to identify the stage of cirrhosis and mortality risk, respectively.9,45 A Child-Pugh grade B classification (seven to nine points) is consistent with early hepatic decompensation,46 whereas a Model for End-Stage Liver Disease score of 12 or more is predictive of increased risk for cirrhosis complications.9
Cirrhosis Management
The primary goals of liver disease management are to prevent cirrhosis complications, liver decompensation, and death. These goals are accomplished with rigorous prevention counseling, monitoring, and management by primary care physicians, in consultation with subspecialists as needed.
PREVENTION COUNSELING
For all patients with liver disease, counseling points should be discussed, including avoidance of alcohol; maintenance of a healthy weight; nutrition; medication and supplement review; prevention of infections (including receiving vaccinations); screening and treatment of causative factors; and avoidance of unnecessary surgical procedures. Table 6 provides more details on counseling for patients with chronic liver disease.7,9,18,21,45,47–52
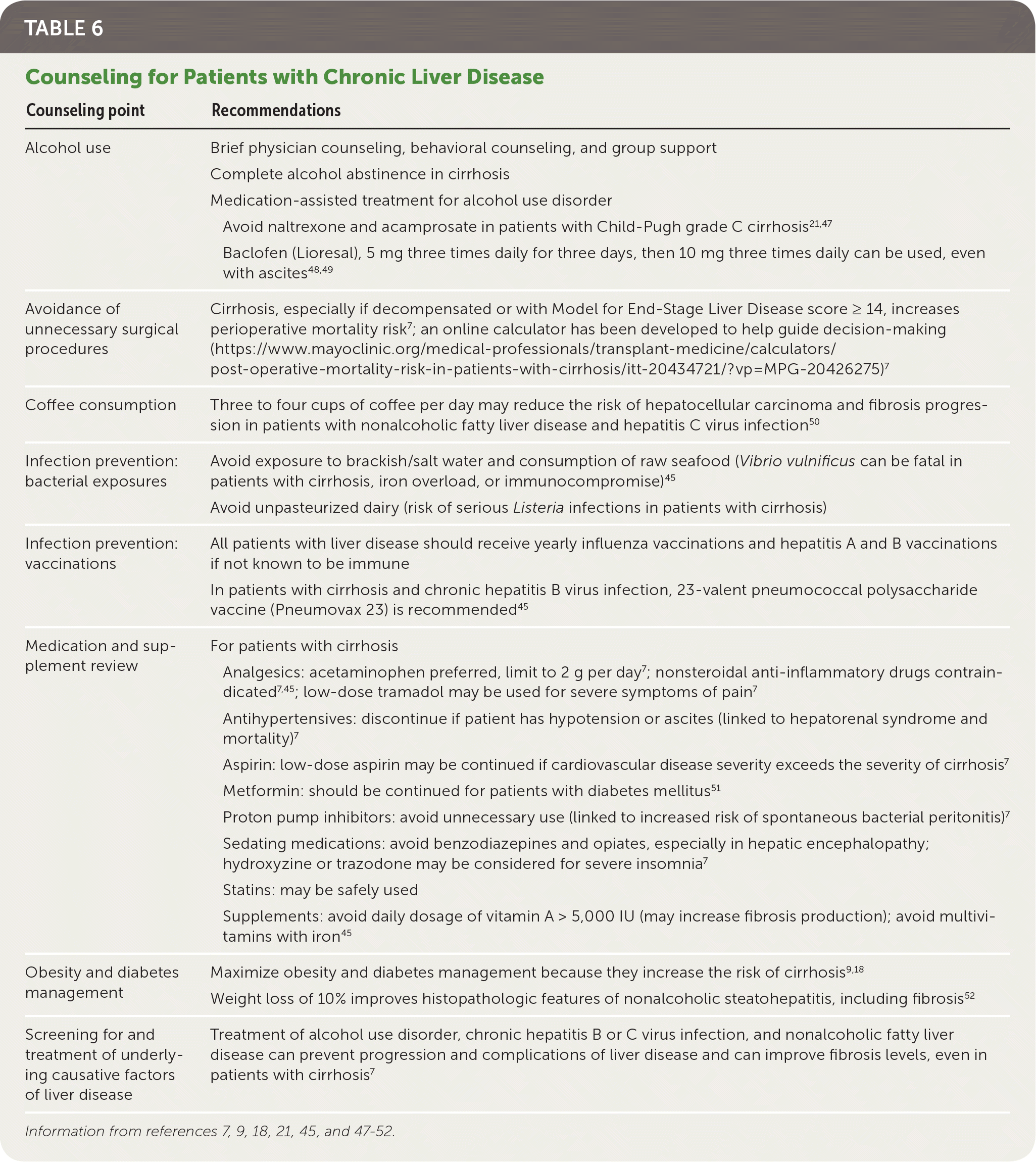
| Counseling point | Recommendations |
|---|---|
| Alcohol use | Brief physician counseling, behavioral counseling, and group support |
| Complete alcohol abstinence in cirrhosis | |
| Medication-assisted treatment for alcohol use disorder | |
| Avoid naltrexone and acamprosate in patients with Child-Pugh grade C cirrhosis21,47 | |
| Baclofen (Lioresal), 5 mg three times daily for three days, then 10 mg three times daily can be used, even with ascites48,49 | |
| Avoidance of unnecessary surgical procedures | Cirrhosis, especially if decompensated or with Model for End-Stage Liver Disease score ≥ 14, increases perioperative mortality risk7; an online calculator has been developed to help guide decision-making (https://www.mayoclinic.org/medical-professionals/transplant-medicine/calculators/post-operative-mortality-risk-in-patients-with-cirrhosis/itt-20434721/?vp=MPG-20426275)7 |
| Coffee consumption | Three to four cups of coffee per day may reduce the risk of hepatocellular carcinoma and fibrosis progression in patients with nonalcoholic fatty liver disease and hepatitis C virus infection50 |
| Infection prevention: bacterial exposures | Avoid exposure to brackish/salt water and consumption of raw seafood (Vibrio vulnificus can be fatal in patients with cirrhosis, iron overload, or immunocompromise)45 |
| Avoid unpasteurized dairy (risk of serious Listeria infections in patients with cirrhosis) | |
| Infection prevention: vaccinations | All patients with liver disease should receive yearly influenza vaccinations and hepatitis A and B vaccinations if not known to be immune |
| In patients with cirrhosis and chronic hepatitis B virus infection, 23-valent pneumococcal polysaccharide vaccine (Pneumovax 23) is recommended45 | |
| Medication and supplement review | For patients with cirrhosis |
| Analgesics: acetaminophen preferred, limit to 2 g per day 7; nonsteroidal anti-inflammatory drugs contraindicated7,45; low-dose tramadol may be used for severe symptoms of pain7 | |
| Antihypertensives: discontinue if patient has hypotension or ascites (linked to hepatorenal syndrome and mortality)7 | |
| Aspirin: low-dose aspirin may be continued if cardiovascular disease severity exceeds the severity of cirrhosis7 | |
| Metformin: should be continued for patients with diabetes mellitus51 | |
| Proton pump inhibitors: avoid unnecessary use (linked to increased risk of spontaneous bacterial peritonitis)7 | |
| Sedating medications: avoid benzodiazepines and opiates, especially in hepatic encephalopathy; hydroxyzine or trazodone may be considered for severe insomnia7 | |
| Statins: may be safely used | |
| Supplements: avoid daily dosage of vitamin A > 5,000 IU (may increase fibrosis production); avoid multivitamins with iron45 | |
| Obesity and diabetes management | Maximize obesity and diabetes management because they increase the risk of cirrhosis9,18 |
| Weight loss of 10% improves histopathologic features of nonalcoholic steatohepatitis, including fibrosis52 | |
| Screening for and treatment of underlying causative factors of liver disease | Treatment of alcohol use disorder, chronic hepatitis B or C virus infection, and nonalcoholic fatty liver disease can prevent progression and complications of liver disease and can improve fibrosis levels, even in patients with cirrhosis7 |
MONITORING OF PATIENTS WITH CIRRHOSIS
For patients with cirrhosis, a basic metabolic panel, liver function tests, complete blood count, and PT/INR should be completed every six months to recalculate Child-Pugh and Model for End-Stage Liver Disease scores. Patients with a Model for End-Stage Liver Disease score of 15 or higher should be referred for liver transplantation evaluation37,45; patients with ascites, hepatic encephalopathy, or variceal hemorrhage should also be referred.37,53
Screening and Management for Specific Complications
Patients with cirrhosis are at risk of multiple complications, including hepatic decompensation, hepatocellular carcinoma, and other more common conditions (e.g., malnutrition, leg cramps, umbilical hernias). Table 7 includes specific recommendations for the screening and management of select complications of cirrhosis.7,9,43,45,46,49,53–55
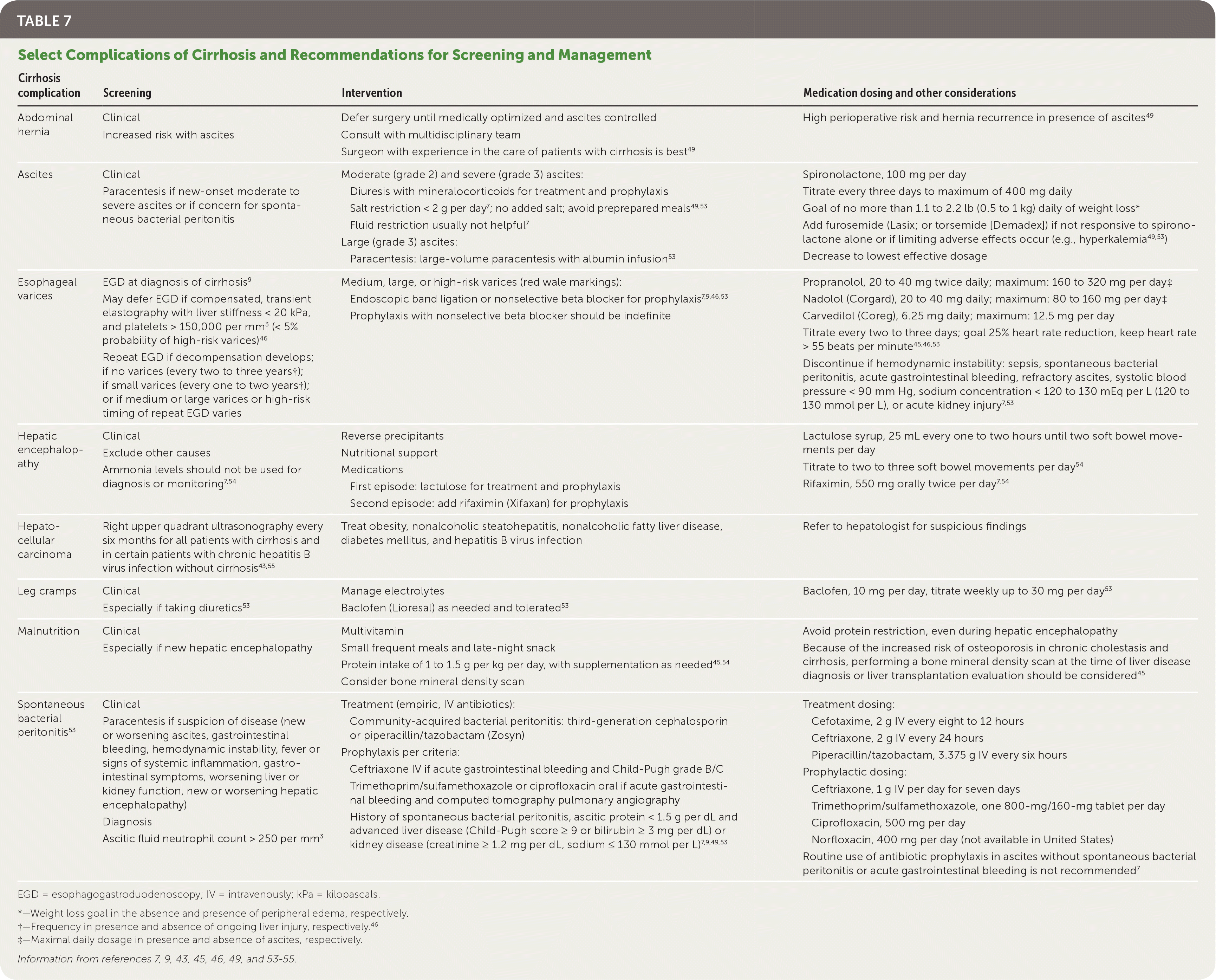
| Cirrhosis complication | Screening | Intervention | Medication dosing and other considerations |
|---|---|---|---|
| Abdominal hernia | Clinical | Defer surgery until medically optimized and ascites controlled | High perioperative risk and hernia recurrence in presence of ascites49 |
| Increased risk with ascites | Consult with multidisciplinary team | ||
| Surgeon with experience in the care of patients with cirrhosis is best49 | |||
| Ascites | Clinical Paracentesis if new-onset moderate to severe ascites or if concern for spontaneous bacterial peritonitis | Moderate (grade 2) and severe (grade 3) ascites: Diuresis with mineralocorticoids for treatment and prophylaxis Salt restriction < 2 g per day 7; no added salt; avoid preprepared meals49,53 Fluid restriction usually not helpful7 Large (grade 3) ascites: Paracentesis: large-volume paracentesis with albumin infusion53 | Spironolactone, 100 mg per day Titrate every three days to maximum of 400 mg daily Goal of no more than 1.1 to 2.2 lb (0.5 to 1 kg) daily of weight loss* Add furosemide (Lasix; or torsemide [Demadex]) if not responsive to spironolactone alone or if limiting adverse effects occur (e.g., hyperkalemia49,53) Decrease to lowest effective dosage |
| Esophageal varices | EGD at diagnosis of cirrhosis9 May defer EGD if compensated, transient elastography with liver stiffness < 20 kPa, and platelets > 150,000 per mm3 (< 5% probability of high-risk varices)46 Repeat EGD if decompensation develops; if no varices (every two to three years†); if small varices (every one to two years†); or if medium or large varices or high-risk timing of repeat EGD varies | Medium, large, or high-risk varices (red wale markings): Endoscopic band ligation or nonselective beta blocker for prophylaxis7,9,46,53 Prophylaxis with nonselective beta blocker should be indefinite | Propranolol, 20 to 40 mg twice daily; maximum: 160 to 320 mg per day‡ Nadolol (Corgard), 20 to 40 mg daily; maximum: 80 to 160 mg per day‡ Carvedilol (Coreg), 6.25 mg daily; maximum: 12.5 mg per day Titrate every two to three days; goal 25% heart rate reduction, keep heart rate > 55 beats per minute45,46,53 Discontinue if hemodynamic instability: sepsis, spontaneous bacterial peritonitis, acute gastrointestinal bleeding, refractory ascites, systolic blood pressure < 90 mm Hg, sodium concentration < 120 to 130 mEq per L (120 to 130 mmol per L), or acute kidney injury 7,53 |
| Hepatic encephalopathy | Clinical | Reverse precipitants | Lactulose syrup, 25 mL every one to two hours until two soft bowel movements per day Titrate to two to three soft bowel movements per day54 Rifaximin, 550 mg orally twice per day 7,54 |
| Exclude other causes | Nutritional support | ||
| Ammonia levels should not be used for diagnosis or monitoring7,54 | Medications First episode: lactulose for treatment and prophylaxis Second episode: add rifaximin (Xifaxan) for prophylaxis | ||
| Hepatocellular carcinoma | Right upper quadrant ultrasonography every six months for all patients with cirrhosis and in certain patients with chronic hepatitis B virus infection without cirrhosis43,55 | Treat obesity, nonalcoholic steatohepatitis, nonalcoholic fatty liver disease, diabetes mellitus, and hepatitis B virus infection | Refer to hepatologist for suspicious findings |
| Leg cramps | Clinical | Manage electrolytes | Baclofen, 10 mg per day, titrate weekly up to 30 mg per day53 |
| Especially if taking diuretics53 | Baclofen (Lioresal) as needed and tolerated53 | ||
| Malnutrition | Clinical | Multivitamin | Avoid protein restriction, even during hepatic encephalopathy Because of the increased risk of osteoporosis in chronic cholestasis and cirrhosis, performing a bone mineral density scan at the time of liver disease diagnosis or liver transplantation evaluation should be considered45 |
| Especially if new hepatic encephalopathy | Small frequent meals and late-night snack | ||
| Protein intake of 1 to 1.5 g per kg per day, with supplementation as needed45,54 | |||
| Consider bone mineral density scan | |||
| Spontaneous bacterial peritonitis53 | Clinical Paracentesis if suspicion of disease (new or worsening ascites, gastrointestinal bleeding, hemodynamic instability, fever or signs of systemic inflammation, gastrointestinal symptoms, worsening liver or kidney function, new or worsening hepatic encephalopathy) Diagnosis Ascitic fluid neutrophil count > 250 per mm3 | Treatment (empiric, IV antibiotics): Community-acquired bacterial peritonitis: third-generation cephalosporin or piperacillin/tazobactam (Zosyn) Prophylaxis per criteria: Ceftriaxone IV if acute gastrointestinal bleeding and Child-Pugh grade B/C Trimethoprim/sulfamethoxazole or ciprofloxacin oral if acute gastrointestinal bleeding and Child-Pugh grade A [corrected] History of spontaneous bacterial peritonitis, ascitic protein < 1.5 g per dL and advanced liver disease (Child-Pugh score ≥ 9 or bilirubin ≥ 3 mg per dL) or kidney disease (creatinine ≥ 1.2 mg per dL, sodium ≤ 130 mmol per L)7,9,49,53 | Treatment dosing: Cefotaxime, 2 g IV every eight to 12 hours Ceftriaxone, 2 g IV every 24 hours Piperacillin/tazobactam, 3.375 g IV every six hours Prophylactic dosing: Ceftriaxone, 1 g IV per day for seven days Trimethoprim/sulfamethoxazole, one 800-mg/160-mg tablet per day Ciprofloxacin, 500 mg per day Norfloxacin, 400 mg per day (not available in United States) Routine use of antibiotic prophylaxis in ascites without spontaneous bacterial peritonitis or acute gastrointestinal bleeding is not recommended7 |
COMMON COMPLICATIONS IN DECOMPENSATED CIRRHOSIS
Ascites, which develops in 5% to 10% of patients with cirrhosis per year, leads to decreased quality of life, frequent hospitalizations, and directly increases risk of further complications such as spontaneous bacterial peritonitis, umbilical hernias, and respiratory compromise. It also portends a poor prognosis, with a 30% five-year survival.53 Hepatic encephalopathy, which occurs in 5% to 25% of patients within five years of a cirrhosis diagnosis, is likewise associated with increased medical cost and mortality, with a reported 15% inpatient mortality rate.54
SCREENING FOR VARICES
Portal hypertension predisposes patients with cirrhosis to develop esophageal varices. Patients with varices have a one in three chance of developing a variceal bleed in the two years after diagnosis, with a 20% to 40% mortality rate per episode.45 Endoscopy is the preferred screening method for esophageal varices. Many experts and guidelines recommend screening all patients with cirrhosis9; however, newer recommendations suggest targeted screening of patients with clinically significant portal hypertension.46 A liver stiffness greater than 20 kPa, alone or combined with a low platelet count (less than 150,000 per mm3) and increased spleen size, and/or the presence of portosystemic collaterals on imaging may be sufficient to diagnose clinically significant portal hypertension and warrant endoscopic screening for varices. Repeat endoscopy should be performed every one to two years if small varices are found and every two to three years if no varices are found.46
Consultation
Varices, hepatic encephalopathy, and ascites herald hepatic decompensation; these conditions warrant referral for subspecialist evaluation. The management of acute or refractory complications of cirrhosis (e.g., spontaneous bacterial peritonitis, acute gastrointestinal bleeding, hepatorenal syndrome, unresponsive portal hypertension, hepatic encephalopathy, ascites) is best addressed in the inpatient or referral setting.
This article updates previous articles on this topic by Starr and Raines,56 Heidelbaugh and Bruderly,57 and Riley and Bhatti.58
Data Sources: A literature search was completed in Medline via Ovid, EBSCOhost, DynaMed, and the Cochrane Database of Systematic Reviews using the keywords cirrhosis, end stage liver disease, management of liver disease, and liver fibrosis staging. Additionally, the EE+Evidence Summary literature search sent by the AFP medical editors was reviewed. Search dates: November 26, 2018; December 27, 2018; and August 7, 2019.
