Am Fam Physician. 2020;101(2):112-114
Related Putting Prevention into Practice: Screening for Hepatitis B Virus Infection in Pregnant Women.
As published by the USPSTF.
Summary of Recommendation and Evidence
The USPSTF recommends screening for hepatitis B virus (HBV) infection in pregnant women at their first prenatal visit (Table 1). A recommendation.
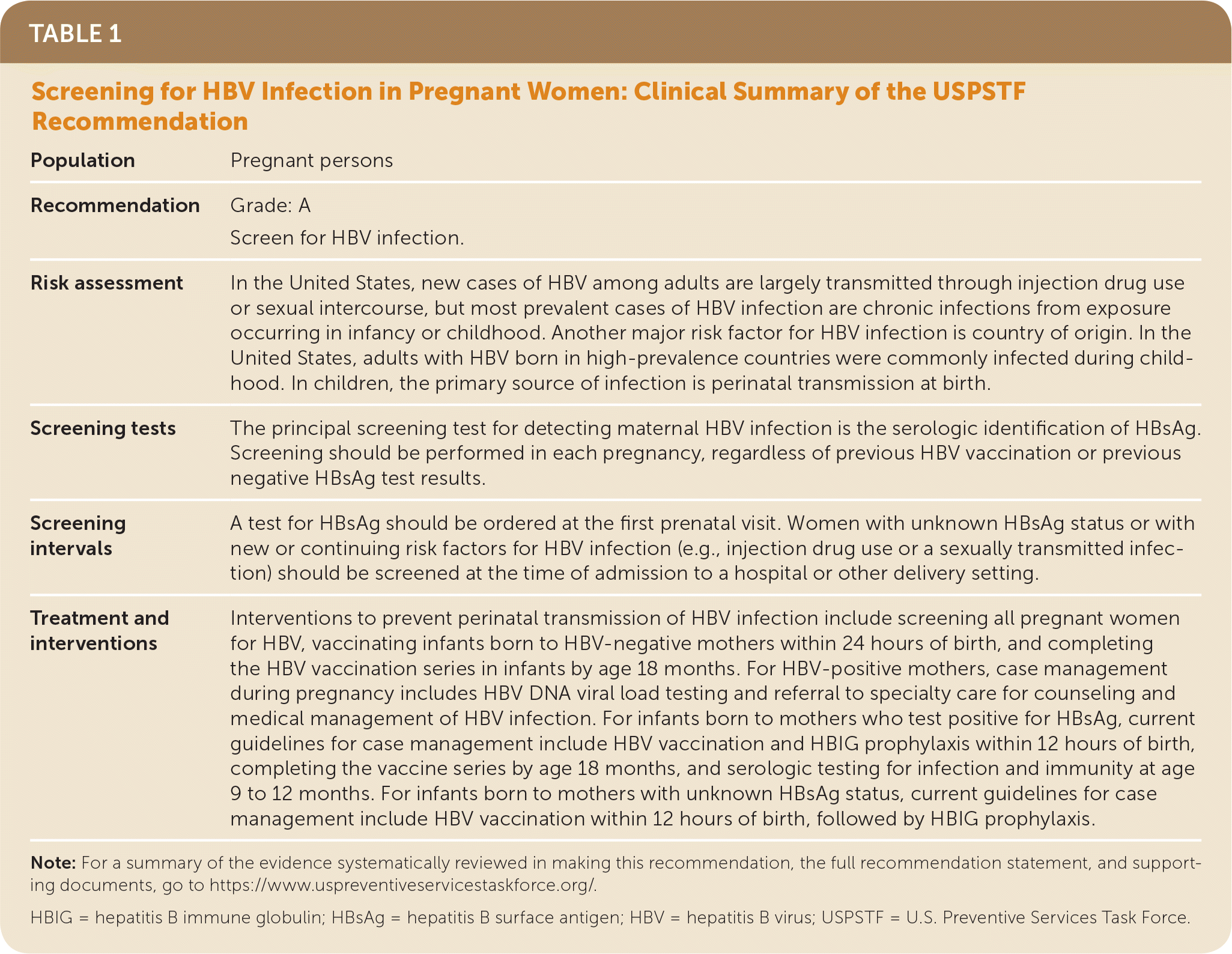
| Population | Pregnant persons |
| Recommendation | Grade: A Screen for HBV infection. |
| Risk assessment | In the United States, new cases of HBV among adults are largely transmitted through injection drug use or sexual intercourse, but most prevalent cases of HBV infection are chronic infections from exposure occurring in infancy or childhood. Another major risk factor for HBV infection is country of origin. In the United States, adults with HBV born in high-prevalence countries were commonly infected during childhood. In children, the primary source of infection is perinatal transmission at birth. |
| Screening tests | The principal screening test for detecting maternal HBV infection is the serologic identification of HBsAg. Screening should be performed in each pregnancy, regardless of previous HBV vaccination or previous negative HBsAg test results. |
| Screening intervals | A test for HBsAg should be ordered at the first prenatal visit. Women with unknown HBsAg status or with new or continuing risk factors for HBV infection (e.g., injection drug use or a sexually transmitted infection) should be screened at the time of admission to a hospital or other delivery setting. |
| Treatment and interventions | Interventions to prevent perinatal transmission of HBV infection include screening all pregnant women for HBV, vaccinating infants born to HBV-negative mothers within 24 hours of birth, and completing the HBV vaccination series in infants by age 18 months. For HBV-positive mothers, case management during pregnancy includes HBV DNA viral load testing and referral to specialty care for counseling and medical management of HBV infection. For infants born to mothers who test positive for HBsAg, current guidelines for case management include HBV vaccination and HBIG prophylaxis within 12 hours of birth, completing the vaccine series by age 18 months, and serologic testing for infection and immunity at age 9 to 12 months. For infants born to mothers with unknown HBsAg status, current guidelines for case management include HBV vaccination within 12 hours of birth, followed by HBIG prophylaxis. |
Rationale
IMPORTANCE
Screening for HBV infection during pregnancy identifies women whose infants are at risk of perinatal transmission. Data from a nationally representative sample showed a prevalence of maternal HBV infection of 85.8 cases per 100,000 deliveries from 1998 to 2011 (0.09% of live-born singleton deliveries in the United States).1,2 Although there are guidelines for universal infant HBV vaccination, rates of maternal HBV infection have increased annually by 5.5% since 1998.1,2 Persons infected with HBV during infancy or childhood are more likely to develop chronic infection. Chronic HBV infection increases long-term morbidity and mortality by predisposing infected persons to cirrhosis of the liver and liver cancer.
REAFFIRMATION
In 2009, the USPSTF reviewed the evidence for screening for HBV infection in pregnant women and issued an A recommendation.3 The USPSTF has decided to use a reaffirmation deliberation process to update this recommendation. The USPSTF uses the reaffirmation process for well-established, evidence-based standards of practice in current primary care practice for which only a very high level of evidence would justify a change in the grade of the recommendation.4 In its deliberation of the evidence, the USPSTF considers whether the new evidence is of sufficient strength and quality to change its previous conclusions about the evidence.
DETECTION
The USPSTF previously reviewed the evidence on serologic testing for HBV (hepatitis B surface antigen [HBsAg]) in pregnancy and found adequate evidence of its accuracy (sensitivity and specificity both > 98%).
BENEFITS OF EARLY DETECTION AND INTERVENTIONS
The USPSTF found convincing evidence that universal prenatal screening for HBV infection substantially reduces perinatal transmission of HBV and the subsequent development of chronic HBV infection. The USPSTF found adequate evidence that vaccination of all infants against HBV infection and providing postexposure prophylaxis with hepatitis B immune globulin (HBIG) at birth to infants of mothers infected with HBV substantially reduce the risk for acquisition of HBV infection in infants.
HARMS OF SCREENING AND INTERVENTIONS
The USPSTF found limited evidence on the harms of screening for HBV infection in pregnant women but bound the potential harms of screening as no greater than small based on the high accuracy of screening and the low likelihood of harms from preventive interventions.
USPSTF ASSESSMENT
Using a reaffirmation process, the USPSTF concludes with high certainty that the net benefit of screening for HBV infection in pregnant women is substantial.
Clinical Considerations
PATIENT POPULATION UNDER CONSIDERATION
This recommendation applies to all pregnant persons.
SCREENING TESTS
The principal screening test for detecting maternal HBV infection is the serologic identification of HBsAg. Screening should be performed in each pregnancy, regardless of previous HBV vaccination or previous negative HBsAg test results.1
SCREENING INTERVAL
A test for HBsAg should be ordered at the first prenatal visit. Women with unknown HBsAg status or with new or continuing risk factors for HBV infection (e.g., injection drug use or a sexually transmitted infection) should be screened at the time of admission to a hospital or other delivery setting.
TREATMENT
Interventions to prevent perinatal transmission of HBV infection include screening all pregnant women for HBV, vaccinating infants born to HBV-negative mothers within 24 hours of birth, and completing the HBV vaccination series in infants by age 18 months. For HBV-positive mothers, case management during pregnancy includes HBV DNA viral load testing and referral to specialty care for counseling and medical management of HBV infection. For infants born to mothers who test positive for HBsAg, current guidelines for case management include HBV vaccination and HBIG prophylaxis within 12 hours of birth, completing the vaccine series, and serologic testing for infection and immunity at age 9 to 12 months. For infants born to mothers with unknown HBsAg status, current guidelines for case management include HBV vaccination within 12 hours of birth, followed by HBIG prophylaxis.5
Emerging evidence has demonstrated that administering tenofovir to HBV-positive women with acute infection significantly reduces the risk of HBsAg seropositivity in infants when combined with HBIG prophylaxis at birth and HBV vaccination.6,7 As a result, recent guidelines recommend testing for viral load, antiviral treatment, and HBV vaccination and HBIG prophylaxis.5
USEFUL RESOURCES
This recommendation statement was first published in JAMA. 2019;322(4):349–354.
The “Other Considerations,” “Discussion,” “Reaffirmation of Previous USPSTF Recommendation,” and “Recommendations of Others” sections of this recommendation statement are available at https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/hepatitis-b-virus-infection-in-pregnant-women-screening.
The USPSTF recommendations are independent of the U.S. government. They do not represent the views of the Agency for Healthcare Research and Quality, the U.S. Department of Health and Human Services, or the U.S. Public Health Service.