Am Fam Physician. 2020;101(8):481-488
Patient information: See related handout on fetal aneuploidy.
Author disclosure: No relevant financial affiliations.
Aneuploidy is the presence of one or more extra chromosomes or the absence of one or more chromosomes. The risk of fetal aneuploidy rises with increasing maternal age. Because fetal aneuploidy can affect any pregnancy, all pregnant women should be offered screening. First-trimester combined screening performed between 10 and 13 weeks' gestation detects 82% to 87% of trisomy 21 (Down syndrome) cases. Second-trimester serum quadruple screening performed between 15 and 22 weeks' gestation detects 81% of trisomy 21 cases. Combinations of these tests include integrated or serum integrated, stepwise sequential, and contingent sequential screenings, all of which improve detection rates compared with each test alone. Fetal cell-free DNA testing (noninvasive prenatal testing) performed at or after 10 weeks' gestation detects more than 99% of trisomy 21 cases, with a lower false-positive rate than traditional first-or second-trimester screening methods. Fetal cell-free DNA testing has similar detection rates in high- and low-risk populations but has lower positive predictive values in younger women. It may be performed as primary screening or as a follow-up test to abnormal findings on first- or second-trimester screenings. Second-trimester ultrasonography has limited utility in aneuploidy screening in women who have already been screened with a first- or second-trimester serum test. Diagnostic tests following a positive screening result include chorionic villus sampling performed between 10 and 13 weeks' gestation or amniocentesis performed after 15 weeks' gestation.
Chromosomal abnormalities affect approximately one in 150 pregnancies1 and are responsible for 50% of early pregnancy losses.2 Aneuploidy is the presence of one or more extra chromosomes or the absence of one or more chromosomes.3 The consequences of fetal aneuploidy vary from incompatibility with life to intellectual and physical disability. Prenatal screening aims to detect the most common forms of aneuploidy compatible with survival beyond early embryologic development into viability. The risk of fetal aneuploidy rises with increasing maternal age. For example, the risk of a woman giving birth to a live newborn with trisomy 21 (Down syndrome) increases from one in 1,480 at 20 years of age to one in 85 at 40 years of age.1 Although the overall birth rate in the United States has declined, the portion of first births to women older than 30 years increased from 23.9% in 2000 to 30.2% in 2014.4,5 Because fetal aneuploidy can affect any pregnancy, all pregnant women should be counseled and offered aneuploidy screening regardless of age.1,6,7
WHAT'S NEW ON THIS TOPIC
Although the overall birth rate in the United States has declined the portion of first births to women older than 30 years increased from 23.9% in 2000 to 30.2% in 2014.
Fetal cell-free DNA testing (noninvasive prenatal testing), which is generally performed at or after 10 weeks' gestation, can be used to determine the likelihood of trisomies 21, 18, and 13, as well as fetal sex and sex chromosome aneuploidy.
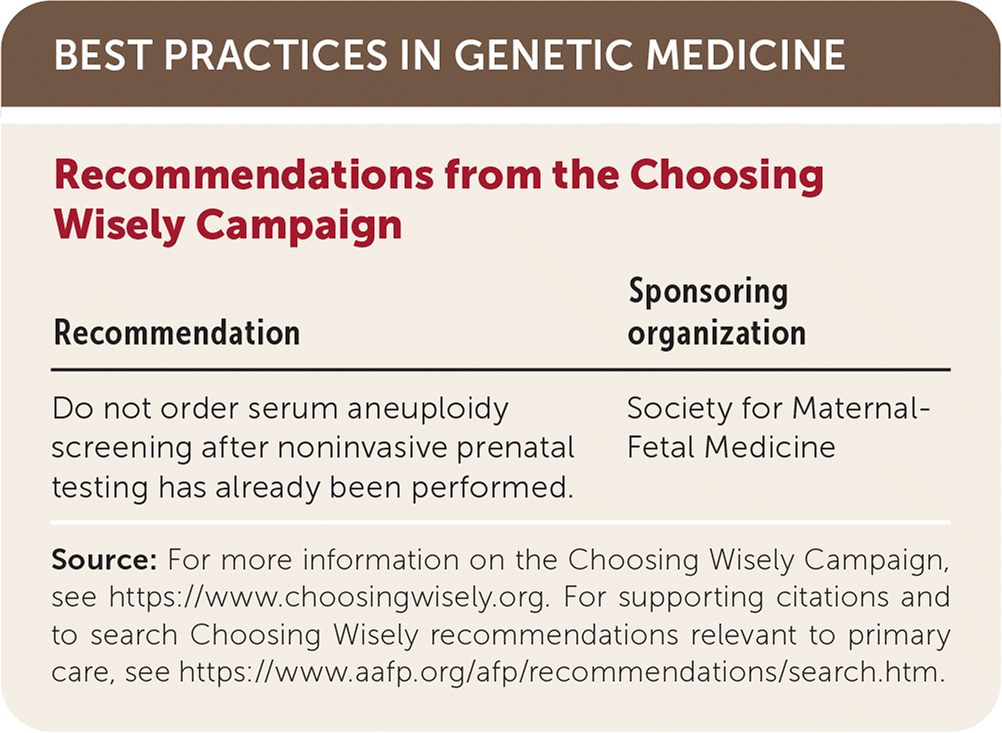
| Recommendation | Sponsoring organization |
|---|---|
| Do not order serum aneuploidy screening after noninvasive prenatal testing has already been performed. | Society for Maternal-Fetal Medicine |
Counseling and Delivering Results
Information from prenatal aneuploidy screening facilitates anticipatory planning and may affect the decision to continue an established pregnancy. Physicians should counsel pregnant women on available screening and diagnostic tests for aneuploidy.8 Counseling should be nondirective, with the physician supporting the autonomy of the woman and her partner in choosing whether to be screened.
Pretest counseling should include a discussion of baseline age-dependent risk, the potential for false-negative and false-positive results, the difference between screening and diagnostic tests, and what types of follow-up testing to expect.9 The use of decision aids (examples are available at https://www.psychosocialresearchgroupunsw.org/decision-aids.html) may improve a woman's ability to make an informed choice.10 All prenatal aneuploidy screening tests optimize detection rates (high sensitivity) and test for relatively uncommon conditions, resulting in high negative predictive values but low positive predictive values. Physicians should communicate test results in a timely manner and discuss the likelihood that a positive result is a true positive. Table 1 defines common terms related to aneuploidy screening.1,9,11
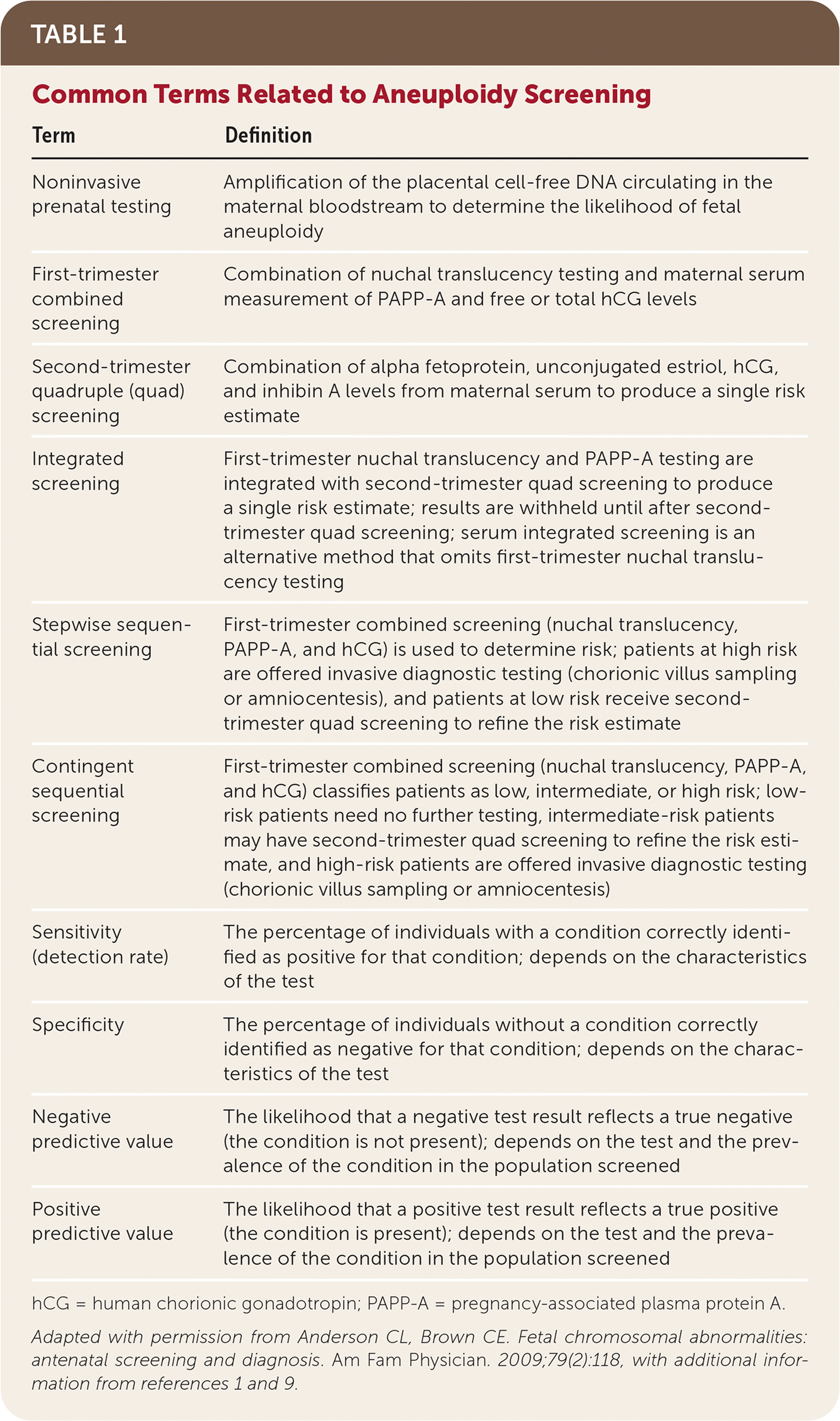
| Term | Definition |
|---|---|
| Noninvasive prenatal testing | Amplification of the placental cell-free DNA circulating in the maternal bloodstream to determine the likelihood of fetal aneuploidy |
| First-trimester combined screening | Combination of nuchal translucency testing and maternal serum measurement of PAPP-A and free or total hCG levels |
| Second-trimester quadruple (quad) screening | Combination of alpha fetoprotein, unconjugated estriol, hCG, and inhibin A levels from maternal serum to produce a single risk estimate |
| Integrated screening | First-trimester nuchal translucency and PAPP-A testing are integrated with second-trimester quad screening to produce a single risk estimate; results are withheld until after second-trimester quad screening; serum integrated screening is an alternative method that omits first-trimester nuchal translucency testing |
| Stepwise sequential screening | First-trimester combined screening (nuchal translucency, PAPP-A, and hCG) is used to determine risk; patients at high risk are offered invasive diagnostic testing (chorionic villus sampling or amniocentesis), and patients at low risk receive second-trimester quad screening to refine the risk estimate |
| Contingent sequential screening | First-trimester combined screening (nuchal translucency, PAPP-A, and hCG) classifies patients as low, intermediate, or high risk; low-risk patients need no further testing, intermediate-risk patients may have second-trimester quad screening to refine the risk estimate, and high-risk patients are offered invasive diagnostic testing (chorionic villus sampling or amniocentesis) |
| Sensitivity (detection rate) | The percentage of individuals with a condition correctly identified as positive for that condition; depends on the characteristics of the test |
| Specificity | The percentage of individuals without a condition correctly identified as negative for that condition; depends on the characteristics of the test |
| Negative predictive value | The likelihood that a negative test result reflects a true negative (the condition is not present); depends on the test and the prevalence of the condition in the population screened |
| Positive predictive value | The likelihood that a positive test result reflects a true positive (the condition is present); depends on the test and the prevalence of the condition in the population screened |
Preimplantation Genetic Screening
Only preimplantation genetic screening performed during the in-vitro fertilization process provides information on aneuploidy before an embryo's implantation in the uterus. Because this type of screening biopsies the portion of an embryo that becomes the placenta, it is susceptible to false-positive and false-negative results attributable to mosaicism (aneuploidy in the placenta that is not present in the fetus).12 Therefore, women who have conceived via in-vitro fertilization and undergone preimplantation genetic screening should still be offered aneuploidy screening during pregnancy.1
Prenatal Screening Tests
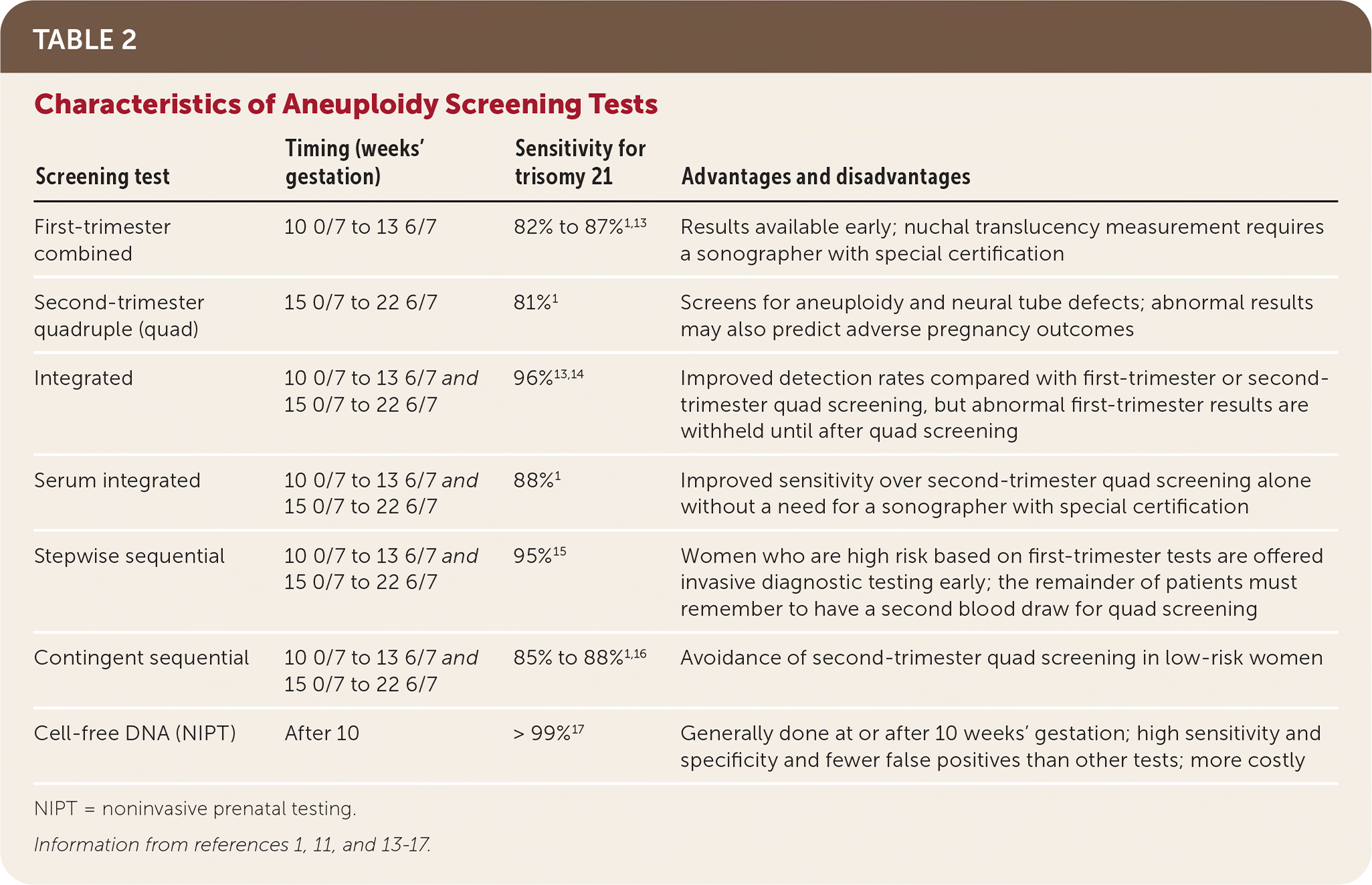
| Screening test | Timing (weeks' gestation) | Sensitivity for trisomy 21 | Advantages and disadvantages |
|---|---|---|---|
| First-trimester combined | 10 0/7 to 13 6/7 | 82% to 87%1,13 | Results available early; nuchal translucency measurement requires a sonographer with special certification |
| Second-trimester quadruple (quad) | 15 0/7 to 22 6/7 | 81%1 | Screens for aneuploidy and neural tube defects; abnormal results may also predict adverse pregnancy outcomes |
| Integrated | 10 0/7 to 13 6/7 and 15 0/7 to 22 6/7 | 96%13,14 | Improved detection rates compared with first-trimester or second-trimester quad screening, but abnormal first-trimester results are withheld until after quad screening |
| Serum integrated | 10 0/7 to 13 6/7 and 15 0/7 to 22 6/7 | 88%1 | Improved sensitivity over second-trimester quad screening alone without a need for a sonographer with special certification |
| Stepwise sequential | 10 0/7 to 13 6/7 and 15 0/7 to 22 6/7 | 95%15 | Women who are high risk based on first-trimester tests are offered invasive diagnostic testing early; the remainder of patients must remember to have a second blood draw for quad screening |
| Contingent sequential | 10 0/7 to 13 6/7 and 15 0/7 to 22 6/7 | 85% to 88%1,16 | Avoidance of second-trimester quad screening in low-risk women |
| Cell-free DNA (NIPT) | After 10 | > 99%17 | Generally done at or after 10 weeks' gestation; high sensitivity and specificity and fewer false positives than other tests; more costly |
FIRST-TRIMESTER SCREENING
First-trimester combined screening consists of ultrasound testing of fetal nuchal translucency, maternal serum pregnancy-associated plasma protein A (PAPP-A) levels, and free or total human chorionic gonadotropin (hCG) levels obtained between 10 0/7 and 13 6/7 weeks' gestation.1,18,19 Nuchal translucency alone should not be used to screen for trisomy 21 in singleton pregnancies. First-trimester combined screening is designed to report 5% of all results as positive, most of which will be false positives. A randomized controlled trial reported a detection rate for trisomy 21 of 87% at 11 weeks' gestation, 85% at 12 weeks, and 82% at 13 weeks.13
Abnormal nuchal translucency is also a predictor of subsequent structural anomalies, and all women with abnormal nuchal translucency should receive detailed ultrasonography at 18 to 22 weeks' gestation.7 The American College of Obstetricians and Gynecologists (ACOG) recommends fetal echocardiography in these cases. 1 Women who choose first-trimester combined screening may still be offered maternal serum alpha fetoprotein measurement between 15 and 22 weeks' gestation (ideally between 16 and 18 weeks) as a screen for open neural tube defects and anencephaly. However, Canadian guidelines suggest that this measurement is unnecessary when high-quality second-trimester ultrasonography is available.7
SECOND-TRIMESTER SCREENING
Second-trimester quadruple (quad) screening includes alpha fetoprotein, unconjugated estriol, hCG, and inhibin A levels from maternal serum. The test is performed between 15 0/7 and 22 6/7 weeks' gestation, although this range may vary slightly by reference laboratory; accurate pregnancy dating is imperative.1,20 Reports will include a baseline risk of trisomies 21 and 18 based on maternal age and the current pregnancy's risk of those trisomies, as well as open spina bifida. As with first-trimester combined screening, laboratories report 5% of all second-trimester quad screening tests as positive, most of which will be false positives. Second-trimester quad screening detects 81% of trisomy 21 cases1 (Table 31,21).
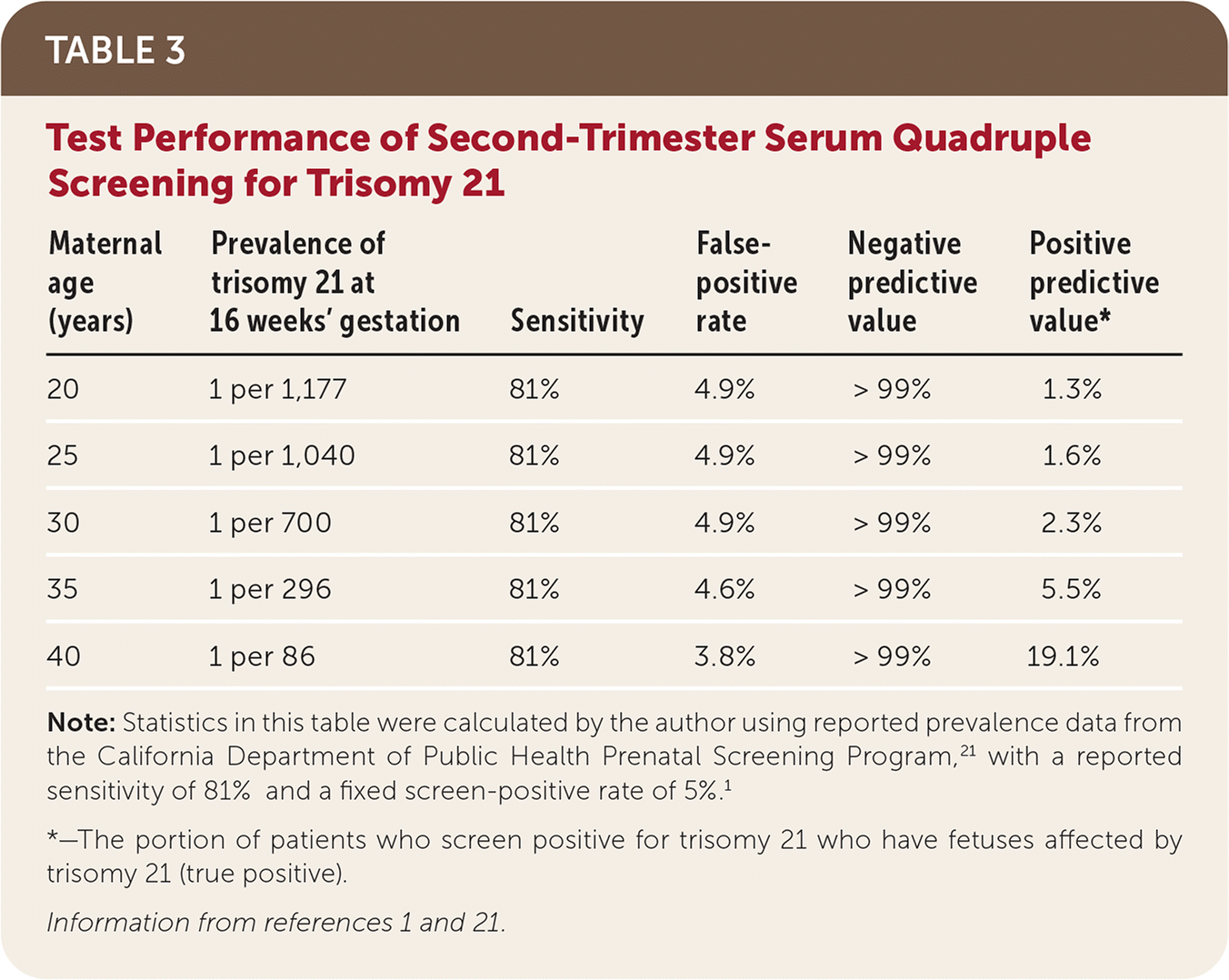
| Maternal age (years) | Prevalence of trisomy 21 at 16 weeks' gestation | Sensitivity | False-positive rate | Negative predictive value | Positive predictive value* |
|---|---|---|---|---|---|
| 20 | 1 per 1,177 | 81% | 4.9% | > 99% | 1.3% |
| 25 | 1 per 1,040 | 81% | 4.9% | > 99% | 1.6% |
| 30 | 1 per 700 | 81% | 4.9% | > 99% | 2.3% |
| 35 | 1 per 296 | 81% | 4.6% | > 99% | 5.5% |
| 40 | 1 per 86 | 81% | 3.8% | > 99% | 19.1% |
A retrospective analysis demonstrated associations between abnormal quad screening markers and adverse pregnancy outcomes.13,22 Women with abnormal quad screening results without subsequent evidence of aneuploidy or neural tube defect may have increased risk of adverse pregnancy outcomes, including preterm birth, fetal growth restriction, preeclampsia, and fetal loss. Increased monitoring for these complications is suggested but has not been shown to improve outcomes.22
COMBINATION FIRST- AND SECOND-TRIMESTER SCREENING
Combinations of first- and second-trimester screening are available to increase the detection rate of trisomy 21.1,13 Integrated screening combines first-trimester maternal serum PAPP-A and fetal nuchal translucency with second-trimester quad screening and detects 96% of trisomy 21 cases.13,14 When performed without first-trimester nuchal translucency (the “serum” integrated screening), the trisomy 21 detection rate is 88%.1 First-trimester results are withheld from the patient until the second-trimester screening is performed.
In stepwise sequential screening, first-trimester combined screening (PAPP-A, hCG, and nuchal translucency) results are given to the patient if positive so that she may be offered early invasive diagnostic testing. When results are negative, quad screening is added in the second trimester to refine risk, resulting in an overall trisomy 21 detection rate of 95%.15
In the contingent sequential screening approach, the results of first-trimester combined screening are classified into three risk categories: high (1% of results), intermediate (18% of results), or low (81% of results).18 Patients at high risk are offered invasive diagnostic testing, and patients at low risk receive no further testing. Patients with intermediate risk are offered second-trimester quad screening to refine risk estimates. Detection rates of 85% to 88% have been reported for this approach.1,16
CELL-FREE DNA TESTING (NIPT)
Placental DNA fragments circulating in the maternal bloodstream are known as fetal cell-free DNA. Cell-free DNA testing, or noninvasive prenatal testing (NIPT), amplifies this DNA to determine if equal amounts are present from each chromosome.23 NIPT, which is generally performed at or after 10 weeks' gestation, can be used to determine the likelihood of trisomies 21, 18, and 13, as well as fetal sex and sex chromosome aneuploidy. It is superior to first- or second-trimester serum screenings with fewer false positives and higher positive predictive values for trisomies 18 and 21.
In a 2015 randomized controlled trial comparing NIPT with first-trimester combined screening, NIPT detected 100% of trisomy 21 cases (false-positive rate of 0.06%) and 78.9% of trisomy 18 cases (false-positive rate of 0.01%).24 A 2017 meta-analysis reported that NIPT had a detection rate of 99.7% for trisomy 21 and 97.9% for trisomy 18, with a false-positive rate of 0.04% for both17 (Table 417,21). Multiple studies have since reported similar or better test performance across low- and high-risk populations.25–28
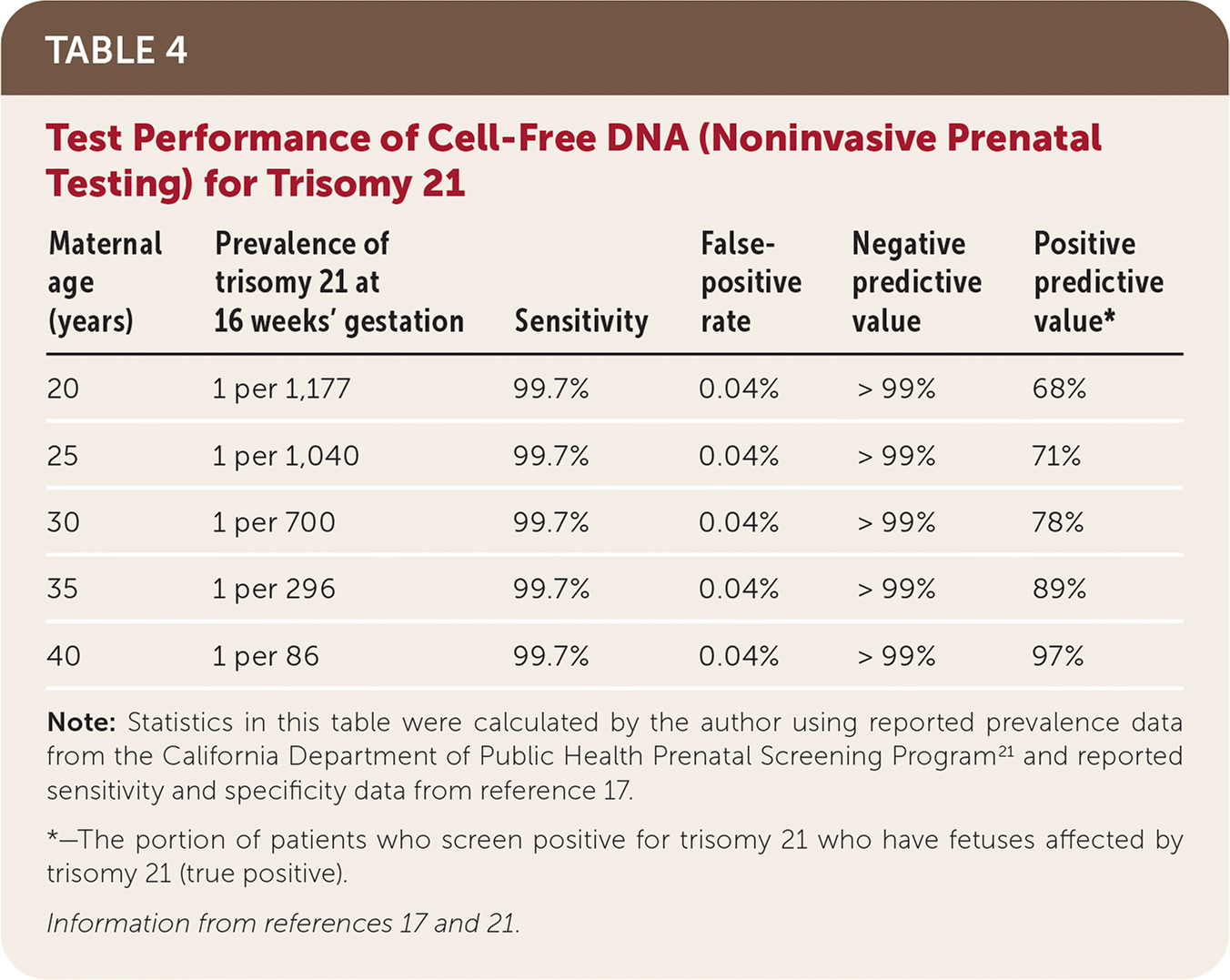
| Maternal age (years) | Prevalence of trisomy 21 at 16 weeks' gestation | Sensitivity | False-positive rate | Negative predictive value | Positive predictive value* |
|---|---|---|---|---|---|
| 20 | 1 per 1,177 | 99.7% | 0.04% | > 99% | 68% |
| 25 | 1 per 1,040 | 99.7% | 0.04% | > 99% | 71% |
| 30 | 1 per 700 | 99.7% | 0.04% | > 99% | 78% |
| 35 | 1 per 296 | 99.7% | 0.04% | > 99% | 89% |
| 40 | 1 per 86 | 99.7% | 0.04% | > 99% | 97% |
NIPT can be performed as primary screening or as a follow-up test when first- or second-trimester serum screening results are abnormal. First- or second-trimester screening should not be performed after NIPT.1 Using NIPT only as a contingent follow-up test avoids invasive testing and its associated risks in most women,29 although some models suggest that as many as one in 50 pregnancies with positive first- or second-trimester screening and normal NIPT results may have an undetected chromosomal abnormality.30 The contingent approach is supported by the Society of Obstetricians and Gynaecologists of Canada.7 ACOG and the Society for Maternal-Fetal Medicine note that NIPT can be used in low-risk populations,1 although positive predictive values are lower. Universal NIPT adoption is not yet cost-effective.31 The Society for Maternal-Fetal Medicine designates some high-risk women as ideal candidates for NIPT screening (risk factors include maternal age of 35 years or older at the time of delivery; ultrasound findings indicating higher risk of aneuploidy; a previous pregnancy affected by trisomy 13, 18, or 21; or positive results from first- or second-trimester serum screenings).32 Positive NIPT results should be confirmed with invasive diagnostic testing, particularly if pregnancy termination is being considered.
Before 10 weeks' gestation, the percentage of fetal vs. maternal cell-free DNA circulating in maternal serum (the fetal fraction) may be too low to create a result. These “no-call” results may indicate an increased risk of aneuploidy.33 Of those women with no-call results, 50% to 80% will receive a reportable result on a repeat test.7,34 Low fetal fraction is more common in pregnant women who are obese, with 7% of women weighing more than 100 kg (220 lb, 7 oz) and 51.1% of women weighing more than 160 kg (352 lb, 12 oz) receiving fetal fractions too low to report at 11 to 13 weeks' gestation.35
Any NIPT test may have a false-positive, false-negative, or no-call result. When abnormal NIPT screening is discordant with (normal) invasive diagnostic testing, it may be attributable to placental mosaicism, maternal aneuploidy, or sometimes occult maternal malignancy. Discordant results, particularly when more than one aneuploidy is seen on NIPT and not confirmed by invasive diagnostic testing, may require a discussion with the patient regarding the risks and benefits of an occult malignancy workup.36,37
TWIN PREGNANCIES
First- and second-trimester serum screening or first-trimester nuchal translucency alone can be used to screen women with twin pregnancies for aneuploidy, although detection rates are lower.1 In higher order pregnancies (triplets or more), serum screening is unvalidated, and only nuchal translucency alone can differentiate which fetus is potentially affected. The Society of Obstetricians and Gynaecologists of Canada notes that NIPT is less validated in twin pregnancies and should be used with caution, and ACOG recommends against it.1,7 However, a meta-analysis of NIPT in twin pregnancies reported a sensitivity of 99% for trisomy 21 and 85% for trisomy 18.38
SECOND-TRIMESTER ULTRASONOGRAPHY
As a stand-alone test, second-trimester ultrasonography has a reported sensitivity of 50% to 60% for trisomy 21.1 A series of “soft markers” for aneuploidy, none of which are considered congenital anomalies, may suggest a higher likelihood of trisomy 21 or 18 when seen on second-trimester ultrasonography.1,39 Many fetuses with aneuploidy will not have these soft markers on ultrasonography, and these soft markers are common in normal fetuses. A meta-analysis found that a thickened nuchal fold is the only soft marker associated with increased risk of trisomy 21.40 When soft markers are isolated, reassurance can be offered to most women after negative quad screening or NIPT testing. The interpretation of isolated soft markers is summarized in Table 5.1,7,41,42 When multiple soft markers are found, referrals to maternal fetal medicine and genetic counseling are warranted.42
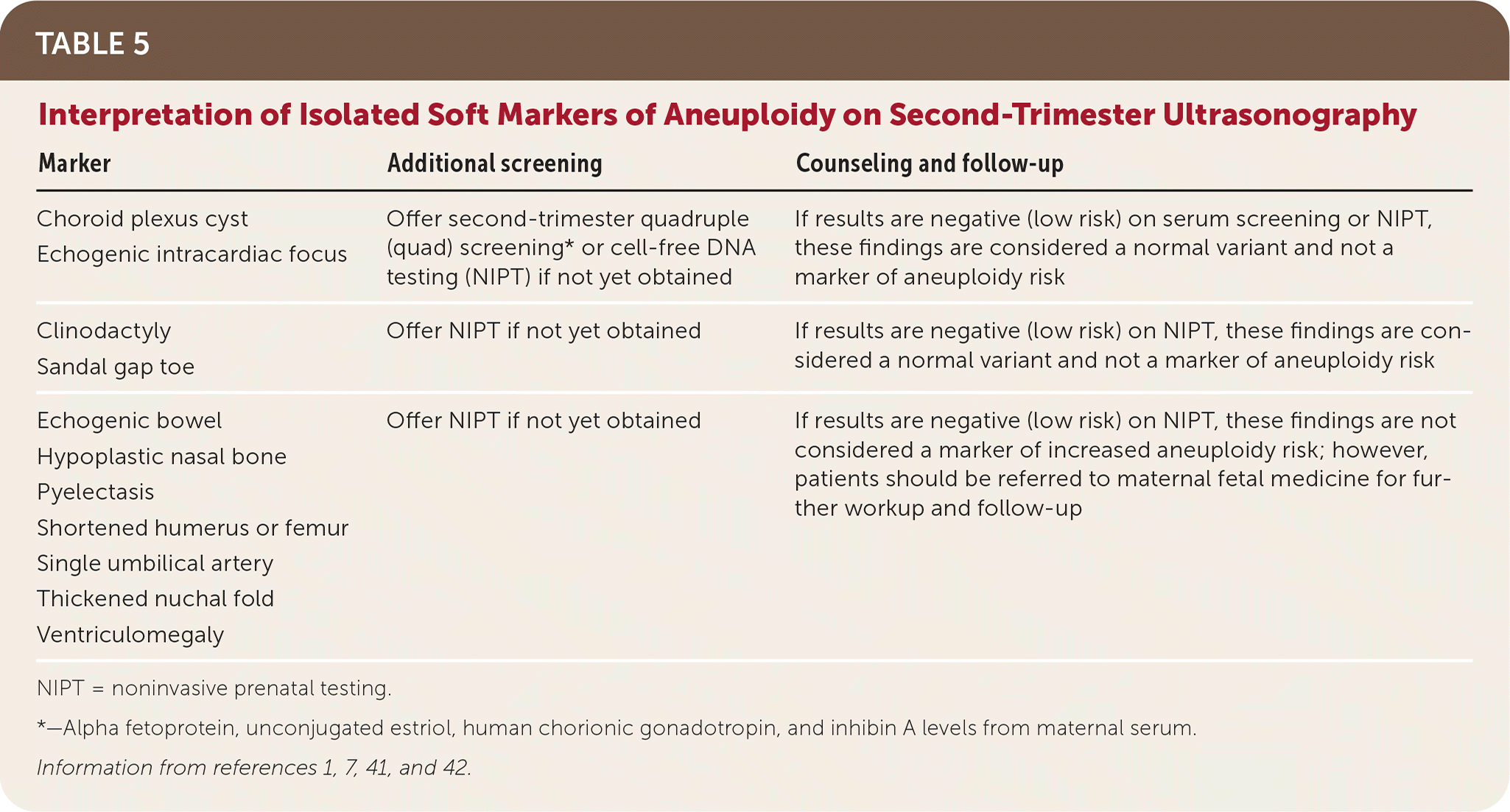
| Marker | Additional screening | Counseling and follow-up |
|---|---|---|
| Choroid plexus cyst Echogenic intracardiac focus | Offer second-trimester quadruple (quad) screening* or cell-free DNA testing (NIPT) if not yet obtained | If results are negative (low risk) on serum screening or NIPT, these findings are considered a normal variant and not a marker of aneuploidy risk |
| Clinodactyly Sandal gap toe | Offer NIPT if not yet obtained | If results are negative (low risk) on NIPT, these findings are considered a normal variant and not a marker of aneuploidy risk |
| Echogenic bowel Hypoplastic nasal bone Pyelectasis Shortened humerus or femur Single umbilical artery Thickened nuchal fold Ventriculomegaly | Offer NIPT if not yet obtained | If results are negative (low risk) on NIPT, these findings are not considered a marker of increased aneuploidy risk; however, patients should be referred to maternal fetal medicine for further workup and follow-up |
Invasive Diagnostic Testing
Women with positive aneuploidy screening results should be offered referral to maternal fetal medicine and genetic counseling to discuss invasive diagnostic testing with chorionic villus sampling or amniocentesis.1,7 Chorionic villus sampling is performed between 10 and 13 weeks' gestation and tests placental tissue obtained transcervically or transabdominally.43 Amniocentesis tests fetal cells grown in a culture from an amniotic fluid sample obtained transabdominally. It is performed any time after 15 weeks' gestation; earlier amniocentesis has higher complication rates.44 Both tests carry a risk of pregnancy loss, with an estimated risk of one in 455 for chorionic villus sampling and one in 900 for amniocentesis.1,45 The laboratory tests performed depend on the indication for the diagnostic procedure but may include karyotyping, chromosomal microarray, or fluorescent in situ hybridization.
This article updates a previous article on this topic by Anderson and Brown.11
Data Sources: The authors searched PubMed for systematic reviews, meta-analyses, and randomized controlled trials involving aneuploidy screening and diagnosis in pregnancy. The TRIP database was queried with similar terms. Relevant guidelines from the Society for Maternal-Fetal Medicine, American College of Obstetricians and Gynecologists, Society of Obstetricians and Gynaecologists of Canada, and Royal College of Obstetricians and Gynaecologists were reviewed. The Cochrane database was also searched. An Essential Evidence Plus summary of patient-oriented evidence that matters was reviewed. Individual references were reviewed from the bibliographies of other specialty guidelines with relevant articles reviewed in full text. Search dates: March 2019 and January 2020.