
Am Fam Physician. 2020;101(9):online
Author disclosure: No relevant financial affiliations.
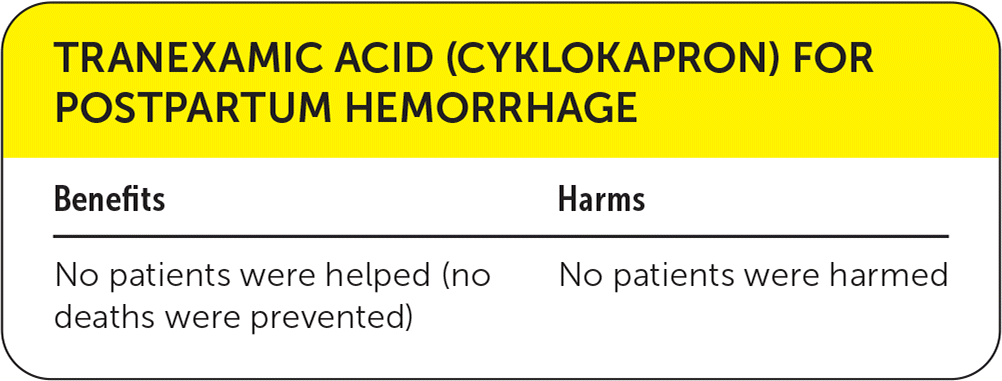
| Benefits | Harms |
|---|---|
| No patients were helped (no deaths were prevented) | No patients were harmed |
Details for This Review
Study Population: 20,060 females 16 years and older with clinical diagnosis of postpartum hemorrhage after vaginal birth or cesarean delivery
Efficacy End Points: Reduction in deaths from all causes, death from bleeding, or hysterectomy
Harm End Points: Thromboembolic events and organ failure
Narrative: Postpartum hemorrhage (PPH) is the most common cause of maternal death worldwide. Tranexamic acid (Cyklokapron) is an antifibrinolytic agent that has been shown to decrease bleeding in surgical patients and all-cause death in trauma patients.1,2 In 2012, tranexamic acid was incorporated into the World Health Organization (WHO) guidelines for refractory or trauma-related PPH.3
The WOMAN trial was a single randomized, double-blind, placebo-controlled, multicenter international study consisting of 20,060 females 16 years and older with a diagnosis of PPH after vaginal birth or cesarean delivery.4 It investigated whether early administration of tranexamic acid reduced the rates of death and hysterectomy in patients with PPH compared with placebo. Outcomes were measured at hospital discharge or on day 42 if the patient was still hospitalized.
Administration of tranexamic acid did not reduce all-cause mortality. However, tranexamic acid showed a reduction in the risk of death due to bleeding (relative risk [RR] = 0.81; 95% CI, 0.62 to 0.98; 0.4% absolute risk difference [ARD]; number needed to treat [NNT] = 267; very low-quality evidence). There was no difference in hysterectomy rate between the two groups.
There was no difference between tranexamic acid and placebo in the rate of thromboembolic events (deep venous thrombosis, pulmonary embolism, myocardial infarction, and stroke), organ failure (renal, cardiac, respiratory), or sepsis and use of uterotonics.
A systematic review and meta-analysis,5 which only included patients with PPH following vaginal delivery and excluded cesarean deliveries (approximately 14,000 patients from two randomized controlled trials, including the subset of patients from the WOMAN trial who had PPH after vaginal delivery), similarly showed no all-cause mortality benefit. However, this meta-analysis showed reduced risk of hysterectomy (RR = 0.63; 95% CI, 0.42 to 0.94; 0.3% ARD; NNT = 333; very low-quality evidence) after PPH resulting from vaginal delivery. This analysis showed that tranexamic acid did not increase the risk of thromboembolic events, stroke, heart attack, or sepsis.
Caveats: The subjective inclusion criteria may have introduced selection bias into the study. Clinical diagnosis of PPH was based on the ability to estimate blood loss (500 mL after vaginal delivery or 1,000 mL during cesarean delivery), which can vary between clinicians. Hemodynamic instability was also an inclusion criteria, but objective measurements to define this were not discussed. Additionally, patients were enrolled only if clinicians were uncertain about giving tranexamic acid, which left out the subgroup of patients with more severe pathology who received tranexamic acid as the standard of care. Excluding these patients, especially those with hemodynamic instability from severe hemorrhage, may have led to an underestimation of the true effectiveness of tranexamic acid.
Another caveat to consider is that the sample size was significantly increased from 15,000 to 20,000 patients after investigators discovered that the decision to perform hysterectomy was commonly made at the time of randomization and, thus, could not be impacted by intervention. This runs the risk of overpowering, which can interpret seemingly small and unremarkable differences between treatment and control arms as statistically significant.
Although we only reported individual outcomes in our review, the original study authors selected a composite outcome (death from all causes or hysterectomy within 42 days of giving birth) as the primary outcome. The authors interpreted the results as positive despite the lack of statistical difference in this primary composite outcome. This was partly from shifting focus from all-cause mortality to cause-specific mortality, specifically death due to bleeding. Although there were significantly fewer deaths from bleeding in the tranexamic acid group, the calculated fragility index was zero, pointing to the lack of robustness of this data set. We also do not consider disease-specific mortality a true effectiveness end point.
In light of the results of this trial, WHO recommends administration of tranexamic acid to all patients with PPH.3 This recommendation is not supported by the existing evidence with regard to all-cause mortality. Therefore, we have assigned a color recommendation of yellow (unclear benefits). However, considering the safety and low cost of tranexamic acid ($45 to $55 per vial), and the potentially devastating consequences of PPH, we believe tranexamic acid should be considered an adjunctive therapy when other modalities (e.g., treating uterine atony) fail to control the hemorrhage.
Copyright © 2020 MD Aware, LLC (theNNT.com). Used with permission.
This series is coordinated by Dean A. Seehusen, MD, MPH, AFP assistant medical editor, and Daniel Runde, MD, from the NNT Group.
A collection of Medicine by the Numbers published in AFP is available at https://www.aafp.org/afp/mbtn.
