
Am Fam Physician. 2020;101(9):551-556
Patient information: See related handout on endometrial biopsy, written by the authors of this article.
Author disclosure: No relevant financial affiliations.
Endometrial biopsy is a safe and efficient method to evaluate the endometrium for a variety of indications, most commonly abnormal uterine bleeding and postmenopausal bleeding. Endometrial biopsy is highly specific for diagnosing atypical hyperplasia and endometrial cancer in postmenopausal women. Pregnancy is the only absolute contraindication to the procedure. The biopsy is performed with an endometrial biopsy catheter that is inserted through the cervix into the uterine cavity. The catheter's piston is then drawn out to create suction. Tissue sampling occurs by rolling the catheter while moving it in and out of the uterine cavity. Nonsteroidal anti-inflammatory drugs can be administered orally before the procedure, and topical lidocaine can be applied to the cervix before starting the procedure to reduce procedure-associated pain. A tenaculum should be applied only if required by cervical mobility or uterocervical angulation because it increases pain and lengthens procedure times. Cramping is a common adverse effect, but serious complications are rare. Patients should be referred for further evaluation if the procedure fails or an insufficient sample is obtained. Postmenopausal women and women with persistent or recurrent symptoms should receive further evaluation even when biopsy results are normal because blind sampling may miss focal lesions.
Endometrial biopsy is a safe, efficient, and cost-effective method for evaluating the endometrium.1,2 This office procedure is commonly performed for evaluation of abnormal uterine bleeding and postmenopausal bleeding; the evaluation may also include imaging studies such as transvaginal ultrasonography, sonohysterography, or hysteroscopy.3,4 In postmenopausal women, an endometrial biopsy is 90% sensitive for endometrial cancer and 82% sensitive for atypical hyperplasia; the specificity is nearly 100% for both.5 Premenopausal women have similar outcomes.6 The current reasonable fair price for this procedure is $750.7

| Recommendation | Sponsoring organization |
|---|---|
| Do not perform endometrial biopsy for the routine evaluation of infertility. | American Society for Reproductive Medicine |
Indications
Premenopausal women with abnormal uterine bleeding who are 45 years or older should have an endometrial biopsy performed as a first-line test.8 Also, biopsy is recommended for women younger than 45 years with abnormal uterine bleeding and risk factors for endometrial hyperplasia (Table 18–10), failed medical management, or persistent bleeding symptoms.4,8

| Age > 50 years Diabetes mellitus Early menarche Hypertension Infertility Late menopause Nulliparity Obesity Personal or family history of endometrial neoplasia Polycystic ovary syndrome Tamoxifen use Thyroid disease Unopposed estrogen therapy |
In postmenopausal women with bleeding but no other risk factors for endometrial hyperplasia or cancer, endometrial biopsy or transvaginal ultrasonography is a first-line approach.3 An ultrasound showing an endometrial thickness of 4 mm or less has a greater than 99% negative predictive value for endometrial cancer and makes further testing unnecessary.3 Endometrial biopsy before transvaginal ultrasonography alters endometrial thickness and creates echogenic spots that can be misinterpreted as sonographic lesions.11 Endometrial biopsy should be the initial evaluation in women with any risk factors for endometrial cancer or hyperplasia3 (Table 18–10). Further evaluation including endometrial biopsy is required for endometrial thickness greater than 4 mm on transvaginal ultrasonography or for any women with persistent or recurrent bleeding.3
Endometrial biopsy is recommended for several clinical situations in the absence of abnormal bleeding. Cervical screening tests that show atypical glandular cells in a patient 35 years or older or who is at risk of endometrial neoplasia or atypical endometrial cells should be followed by endometrial biopsy.12 Endometrial sampling is recommended as surveillance for women receiving nonsurgical management for endometrial hyperplasia.13 Women with hereditary nonpolyposis colorectal cancer (formerly known as Lynch syndrome) are advised to have endometrial biopsy perfomed every one to two years starting at 30 to 35 years of age because of an elevated lifetime risk of endometrial cancer of up to 61%.14 Table 2 summarizes the indications for endometrial biopsy.8–10,12,14,15
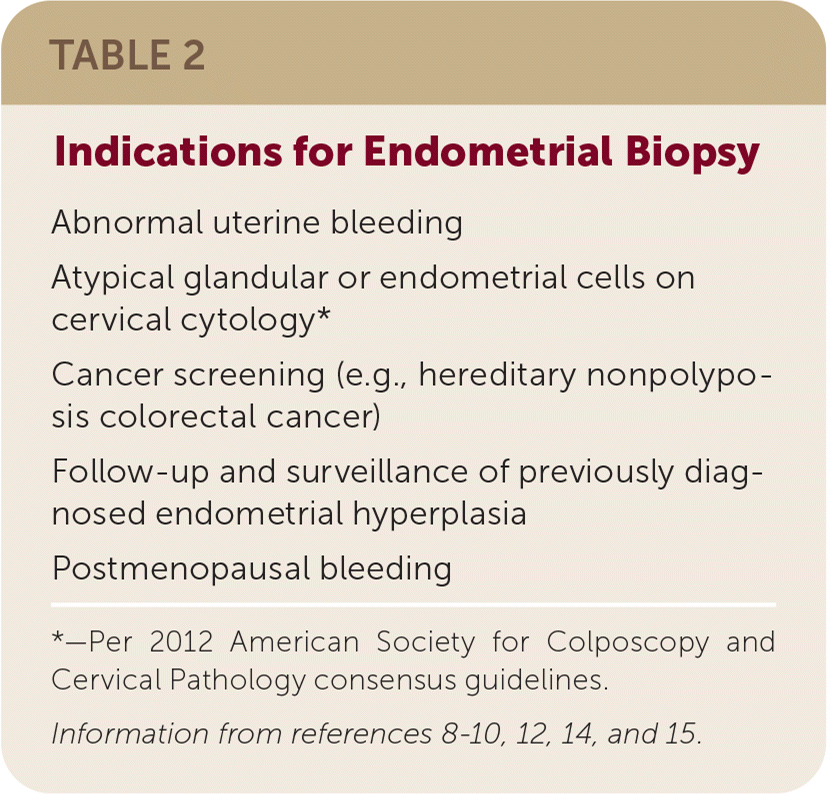
| Abnormal uterine bleeding Atypical glandular or endometrial cells on cervical cytology* Cancer screening (e.g., hereditary nonpolyposis colorectal cancer) Follow-up and surveillance of previously diagnosed endometrial hyperplasia Postmenopausal bleeding |
The American Society for Reproductive Medicine, as part of the Choosing Wisely campaign, recommends against endometrial biopsy in the routine evaluation of infertility.16 Incidentally discovered endometrial measurements greater than 4 mm on transvaginal ultrasonography in postmenopausal women without bleeding should not automatically prompt a biopsy; instead, an assessment of the risk of endometrial hyperplasia and cancer should be completed.3
Contraindications
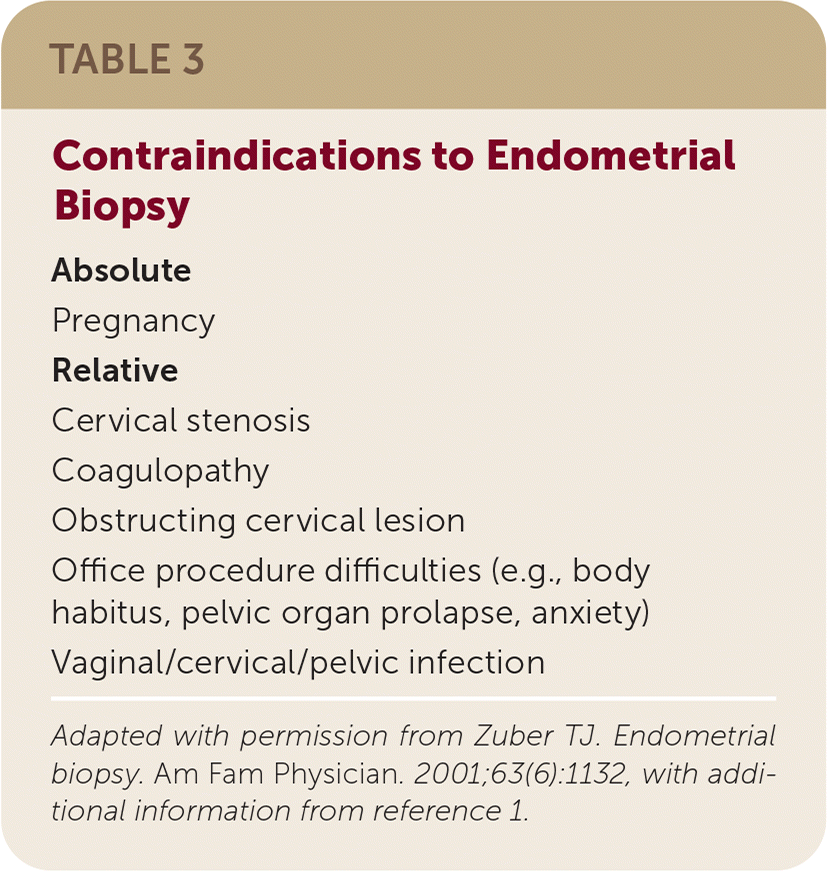
| Absolute Pregnancy Relative Cervical stenosis Coagulopathy Obstructing cervical lesion Office procedure difficulties (e.g., body habitus, pelvic organ prolapse, anxiety) Vaginal/cervical/pelvic infection |
Preprocedure Care
A patient history confirming the indication and excluding contraindications to the procedure should be taken. Antibiotic prophylaxis is not recommended, even for patients with known valvular heart disease.18 Patients may be instructed to take a dose of an oral nonsteroidal anti-inflammatory drug 30 to 60 minutes before the procedure to reduce discomfort from uterine cramping.19,20 Routine use of oral misoprostol (Cytotec) is not recommended because it increases procedural adverse effects including uterine cramping, nausea, diarrhea, and abdominal pain without increasing ease or success of the biopsy.21,22 Misoprostol may be helpful in patients with cervical stenosis.
Procedure
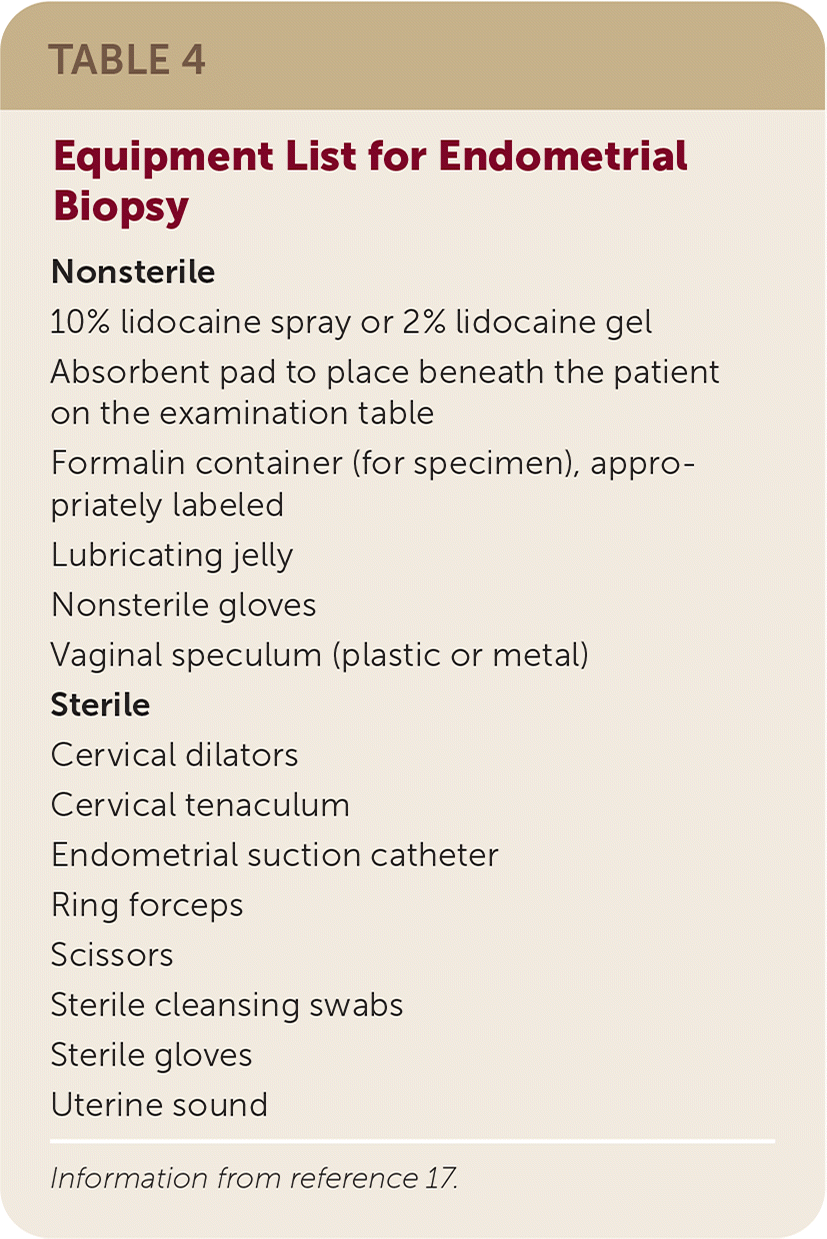
| Nonsterile 10% lidocaine spray or 2% lidocaine gel Absorbent pad to place beneath the patient on the examination table Formalin container (for specimen), appropriately labeled Lubricating jelly Nonsterile gloves Vaginal speculum (plastic or metal) Sterile Cervical dilators Cervical tenaculum Endometrial suction catheter Ring forceps Scissors Sterile cleansing swabs Sterile gloves Uterine sound |
With the patient in the lithotomy position, a bimanual examination is performed wearing nonsterile gloves to determine the size and position of the uterus. Insertion of an appropriately sized speculum helps to visualize and center the cervix.
The cervix may be anesthetized with topical anesthetics (e.g., 10% lidocaine spray, 2% lidocaine gel) three minutes before starting the procedure to reduce pain from cervical manipulation and uterine contractions.23 One study using topical benzocaine did not demonstrate a reduction in pain scores; however, recent trials have found a significant reduction in procedure-associated pain among patients treated with topical lidocaine.23–27 The cervix should be cleansed with an antiseptic solution using a ringed forceps to assist with the application.
Sterile gloves should be worn for the procedure. The physician probes the cervix with a sterile uterine sound to assess the uterine depth and direction. The depth, typically 6 to 8 cm, is indicated by the gradations on the sound. Shorter measured lengths suggest that the sound has not passed the internal cervical os. Steady, gentle pressure is typically required to insert the sound through a closed internal os. This step, and the subsequent biopsy, often may be accomplished without the use of a tenaculum.28 Using a tenaculum increases pain and is associated with longer procedure times.28 If the cervix is too mobile or the uterocervical angle too marked, a tenaculum should be applied to the anterior lip of the cervix, then gently pulled outward to stabilize the cervix and straighten the uterocervical angle. The patient should be warned before the tenaculum is placed. If insertion is unsuccessful, a cervical dilator may be used to open the cervical os.
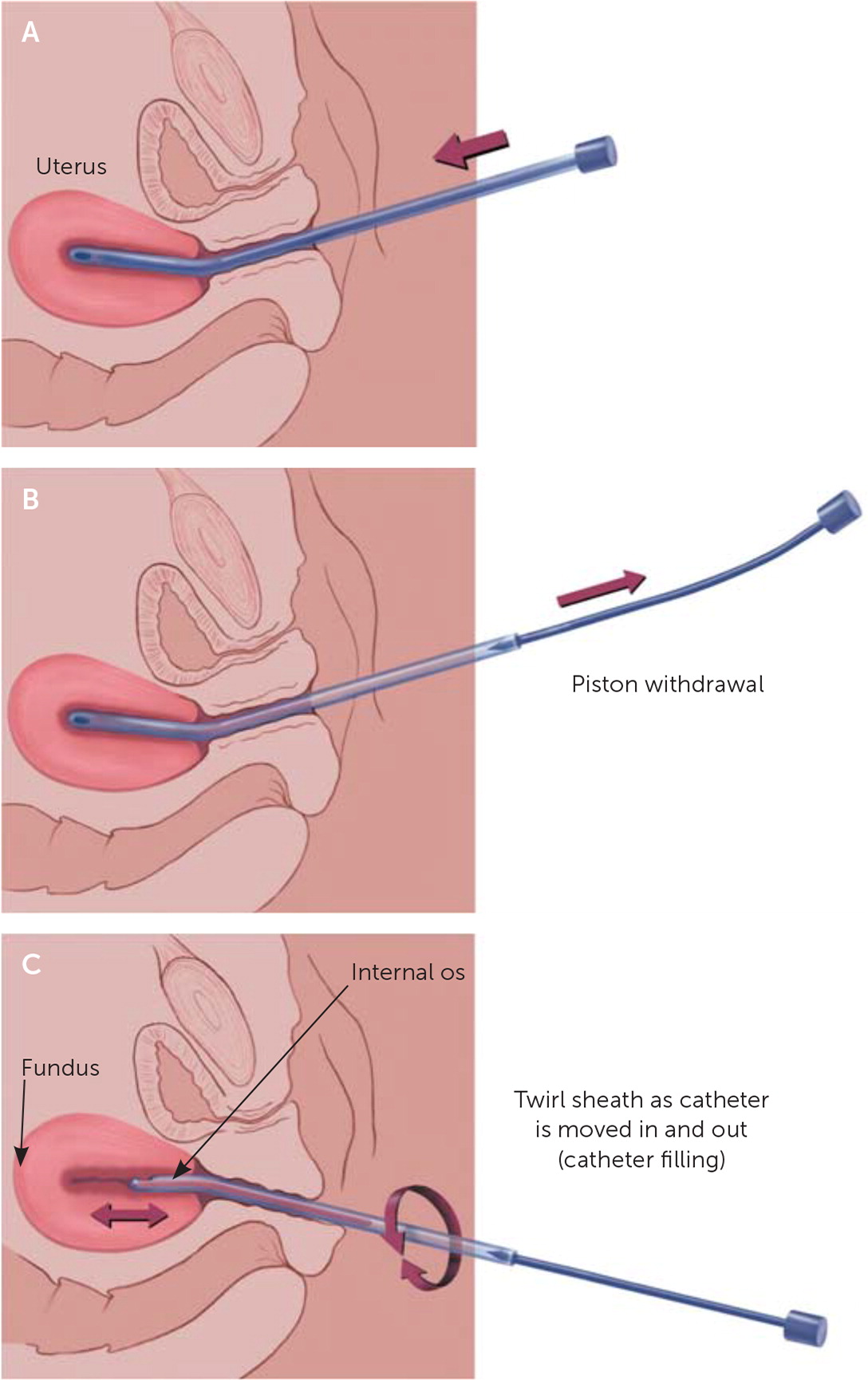
Next, the physician should insert the endometrial biopsy catheter through the cervical canal and advance it to the uterine fundus according to the depth obtained from sounding. The physician should avoid contamination from vaginal tissue. A pressure release may be detected after passing through the internal cervical os, and the next resistance felt will be the fundus. Traction on the tenaculum, if used, should then be released. With the sheath positioned within the uterine cavity and stabilized with the thumb and index finger of one hand, the internal piston on the catheter is rapidly and fully withdrawn the full permissible distance to create suction at the catheter tip. The catheter is then continuously rotated 360 degrees by rolling or twirling the sheath between two fingers while simultaneously moving the catheter between the uterine fundus and the internal cervical os using an in and out motion (Figure 1).17 To maintain the vacuum within the sample pipelle, the physician must ensure that the distal tip containing the curette opening does not exit the endometrial cavity. Three or four up and down passes are recommended to obtain adequate tissue and reduce the probability of an insufficient sample for diagnosis.
When the catheter is filled with tissue, the physician fully withdraws the device, taking care not to contaminate the tip. The tip of the catheter can be cut off using scissors to aid in expelling the sample, but this is not required. The piston is fully advanced into the sheath to evacuate the sample into a formalin container. Biopsy material will appear as a dark red tissue core and should not disintegrate in formalin. Material that disintegrates in the sample container is likely blood instead of endometrial tissue. If the sample appears insufficient, the procedure may be repeated using either a new catheter or the original one if it is intact and uncontaminated.
The tenaculum is carefully removed, and the cervix is observed for bleeding at the site of placement. Bleeding can be stopped with pressure using a cotton swab, or with silver nitrate or ferric subsulfate added if necessary. Before removal of the vaginal speculum, all residual blood and antiseptic cleansing solution should be cleaned from the cervix and vaginal vault.
Postprocedure Care and Complications
The patient should stay in a semirecumbent position for a few minutes to minimize the likelihood of a vasovagal reaction. If the patient is not light-headed or reporting heavy bleeding, she can be discharged. Cramping may be managed with nonsteroidal anti-inflammatory drugs. The patient should be instructed to report any fever, persistent cramping, abdominal pain, or bleeding heavier than a period occurring within 24 to 48 hours of the biopsy.9
Results and Follow-up
Insufficient samples are common in postmenopausal women. Insufficient samples have been reported in an average of 31% of samples, with studies reporting rates between 1% and 53%. A failure to obtain any tissue occurs in 11% of samples.5 Contributing factors for insufficient samples include cervical stenosis, uterine prolapse, focal endometrial pathology (e.g., uterine polyps, submucosal fibroids), and endometrial atrophy.1,5 A patient should be referred for further evaluation if office sampling fails or is inadequate.5
No further evaluation is indicated for normal biopsy results in premenopausal women.8,9,17 Further evaluation is recommended in all women with postmenopausal bleeding and a normal biopsy because of low sampling accuracy.5 Blind sampling may miss focal lesions; therefore, further evaluation is needed when symptoms persist or recur despite a normal biopsy result.5,8
Data Sources: A PubMed search was completed using the key words endometrial biopsy, endometrial sampling, abnormal uterine bleeding, postmenopausal bleeding, dysfunctional uterine bleeding, and endometrial cancer. Additional searches included the Cochrane database, Agency for Healthcare Research and Quality, Essential Evidence Plus, and the U.S. Preventive Services Task Force. Search dates: February 2019, July 2019, and February 2020.
The contents of this article are solely the views of the authors and do not necessarily represent the official views of the Uniformed Services University of the Health Sciences, the U.S. Air Force, the U.S. Army, the U.S. Navy, the U.S. military at large, the U.S. Department of Defense, or the U.S. government.
