
Am Fam Physician. 2020;101(9):557-559
Related practice guideline: Pharmacologic Management of COPD Exacerbations: A Clinical Practice Guideline from the AAFP
Author disclosure: No relevant financial affiliations.
Key Clinical Issue
What are the benefits and harms of pharmacologic and nonpharmacologic treatments for exacerbations of chronic obstructive pulmonary disease (COPD) in adults?
Evidence-Based Answer
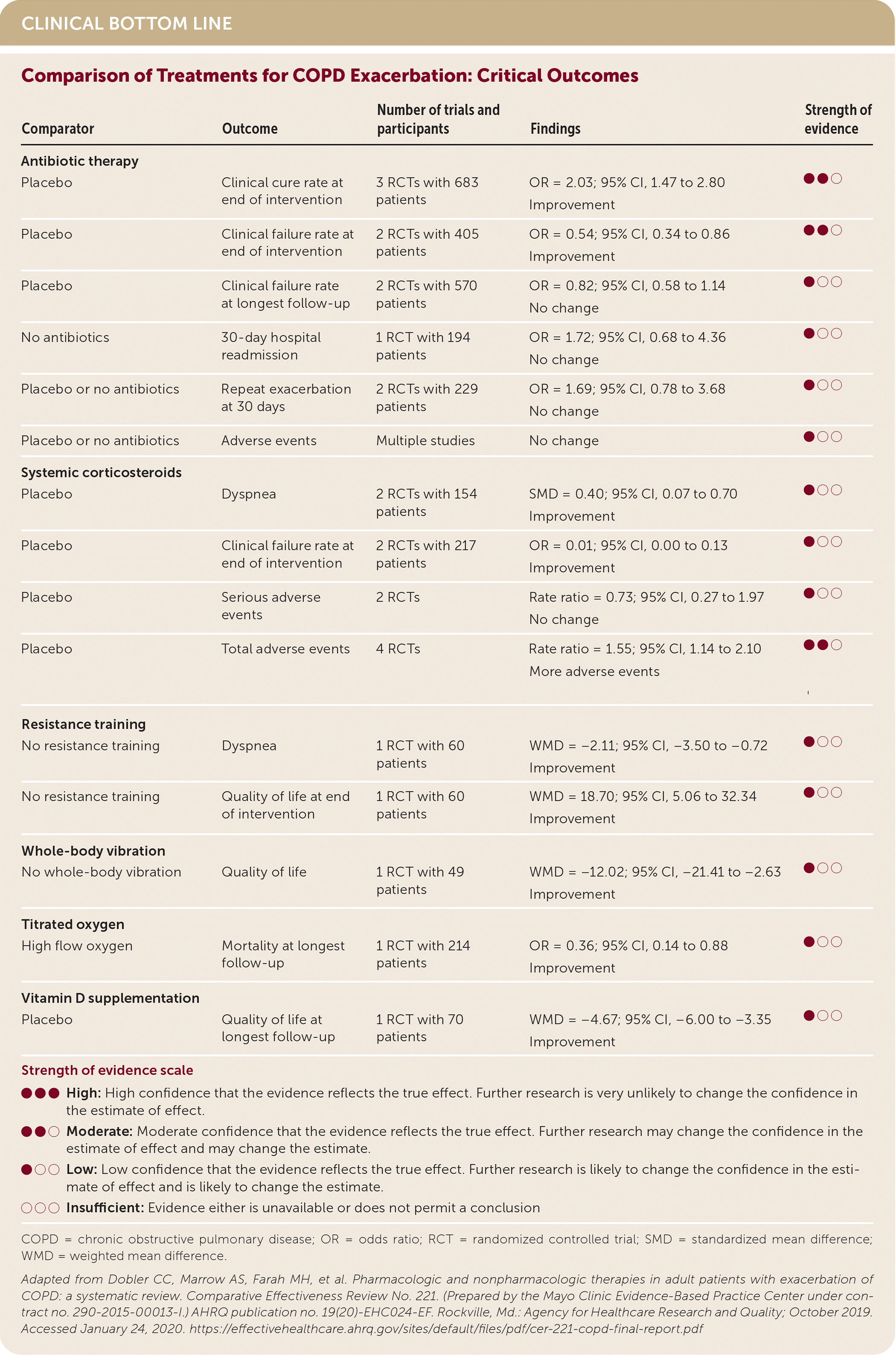
In adults with exacerbations of COPD, antibiotic therapy increases the clinical cure rate and decreases the clinical failure rate. (Strength of Recommendation [SOR]: A, based on consistent, good-quality patient-oriented evidence.) Systemic corticosteroid therapy improves dyspnea and reduces the clinical failure rate. (SOR: B, based on inconsistent or limited-quality patient-oriented evidence.) Titrated oxygen reduces mortality when compared with high flow oxygen. (SOR: B, based on inconsistent or limited-quality patient-oriented evidence.) Resistance training improves dyspnea and quality of life. (SOR: B, based on inconsistent or limited-quality patient-oriented evidence.) Early pulmonar y rehabilitation initiated during hospitalization improves dyspnea.1 (SOR: B, based on inconsistent or limited-quality patient-oriented evidence.)
Practice Pointers
COPD affects 10% of the global population. In the United States, it affects 15 million people, is the fourth leading cause of death, and costs the health care system $32 billion each year.1 COPD is a chronic disease marked by exacerbations that impact quality of life and are potentially fatal. This Agency for Healthcare Research and Quality (AHRQ) review was conducted to evaluate the effectiveness and harms of pharmacologic and nonpharmacologic treatments for COPD exacerbations in adults.1
The review included 98 randomized controlled trials (RCTs) encompassing 13,401 patients. When compared with placebo, antibiotic therapy improved the clinical cure rate (three RCTs with 683 patients; odds ratio [OR] = 2.03; 95% CI, 1.47 to 2.80) and decreased the clinical failure rate by the end of the intervention (two RCTs with 405 patients; OR = 0.54; 95% CI, 0.34 to 0.86). Clinical cure was defined as improvement in signs and symptoms; clinical failure was defined as a lack of improvement in signs and symptoms or the need for additional treatment. There were no differences in clinical failure rates at the longest follow-up, hospital readmissions at 30 days, repeat exacerbations at 30 days, or adverse events. Multiple comparisons among antibiotics, doses, and durations were mostly insufficient for showing an improvement in clinical cure and failure rates, repeat exacerbations, and mortality.
In general, systemic corticosteroids were more effective than placebo in improving dyspnea on a numeric scale (two RCTs with 154 patients; standardized mean difference = 0.40; 95% CI, 0.07 to 0.70) and reducing clinical failure rate at the end of the intervention (two RCTs with 217 patients; OR = 0.01; 95% CI, 0.00 to 0.13). Some evidence indicated that total adverse events were higher with systemic corticosteroids compared with placebo (rate ratio = 1.55; 95% CI, 1.14 to 2.10), but there was no difference in serious adverse events such as gastrointestinal bleeding, hypertension, or psychiatric disorders. The evidence was insufficient to compare different corticosteroids with respect to dyspnea, clinical failure, and death. Evidence was insufficient to compare different routes of administration and duration of corticosteroid use. The evidence was insufficient to determine the effects of other pharmacologic agents, including inhaled antibiotics, inhaled corticosteroids, magnesium, and mucolytics.
| Comparator | Outcome | Number of trials and participants | Findings | Strength of evidence |
|---|---|---|---|---|
| Antibiotic therapy | ||||
| Placebo | Clinical cure rate at end of intervention | 3 RCTs with 683 patients | OR = 2.03; 95% CI, 1.47 to 2.80 Improvement | ● ● ○ |
| Placebo | Clinical failure rate at end of intervention | 2 RCTs with 405 patients | OR = 0.54; 95% CI, 0.34 to 0.86 Improvement | ● ● ○ |
| Placebo | Clinical failure rate at longest follow-up | 2 RCTs with 570 patients | OR = 0.82; 95% CI, 0.58 to 1.14 No change | ● ○ ○ |
| No antibiotics | 30-day hospital readmission | 1 RCT with 194 patients | OR = 1.72; 95% CI, 0.68 to 4.36 No change | ● ○ ○ |
| Placebo or no antibiotics | Repeat exacerbation at 30 days | 2 RCTs with 229 patients | OR = 1.69; 95% CI, 0.78 to 3.68 No change | ● ○ ○ |
| Placebo or no antibiotics | Adverse events | Multiple studies | No change | ● ○ ○ |
| Systemic corticosteroids | ||||
| Placebo | Dyspnea | 2 RCTs with 154 patients | SMD = 0.40; 95% CI, 0.07 to 0.70 Improvement | ● ○ ○ |
| Placebo | Clinical failure rate at end of intervention | 2 RCTs with 217 patients | OR = 0.01; 95% CI, 0.00 to 0.13 Improvement | ● ○ ○ |
| Placebo | Serious adverse events | 2 RCTs | Rate ratio = 0.73; 95% CI, 0.27 to 1.97 No change | ● ○ ○ |
| Placebo | Total adverse events | 4 RCTs | Rate ratio = 1.55; 95% CI, 1.14 to 2.10 More adverse events | ● ● ○ |
| Resistance training | ||||
| No resistance training | Dyspnea | 1 RCT with 60 patients | WMD = −2.11; 95% CI, −3.50 to −0.72 Improvement | ● ○ ○ |
| No resistance training | Quality of life at end of intervention | 1 RCT with 60 patients | WMD = 18.70; 95% CI, 5.06 to 32.34 Improvement | ● ○ ○ |
| Whole-body vibration | ||||
| No whole-body vibration | Quality of life | 1 RCT with 49 patients | WMD = −12.02; 95% CI, −21.41 to −2.63 Improvement | ● ○ ○ |
| Titrated oxygen | ||||
| High flow oxygen | Mortality at longest follow-up | 1 RCT with 214 patients | OR = 0.36; 95% CI, 0.14 to 0.88 Improvement | ● ○ ○ |
| Vitamin D supplementation | ||||
| Placebo | Quality of life at longest follow-up | 1 RCT with 70 patients | WMD = −4.67; 95% CI, −6.00 to −3.35 Improvement | ● ○ ○ |
Resistance training involving the upper and lower body with progressively increased elastic band tension and number of exercise repetitions for nine days was helpful in relieving dyspnea (one RCT with 60 patients; weighted mean difference [WMD] = −2.11 on the 10-point Modified Borg scale, small effect; 95% CI, −3.50 to −0.72) and improving quality of life (one RCT with 60 patients; WMD = 18.70 on the 100-point EQ-5D visual analog scale, small effect; 95% CI, 5.06 to 32.34) when compared with no resistance training. Evidence was insufficient to demonstrate a benefit of resistance training with respect to hospital readmissions and mortality.
Whole-body vibration improved quality of life (one RCT with 49 patients; WMD = −12.02 on the 100-point St. George's Respiratory Questionnaire, small effect; 95% CI, −21.41 to −2.63) when compared with no vibration. Titrated oxygen (to keep oxygen saturation between 88% and 92%) improved prehospital and in-hospital mortality at the longest follow-up (one RCT with 214 patients; OR = 0.36; 95% CI, 0.14 to 0.88) when compared with high flow oxygen. There was no evidence of benefit associated with chest physiotherapy. Early pulmonary rehabilitation initiated during hospitalization for exacerbation was found to improve six-minute walking difference (three RCTs with 253 patients; WMD = 20.02; 95% CI, 12.06 to 28.67), but there was not enough evidence to show a readmission or mortality benefit. There was no significant difference in adverse events between those who received nonpharmacologic therapies and those who did not.
This AHRQ review emphasizes many therapies that family physicians are already using for their patients experiencing COPD exacerbations, such as antibiotics and corticosteroid therapy, while also suggesting that magnesium and mucolytics may not be helpful. Nonpharmacologic therapies such as resistance training and whole-body vibration may be beneficial. The American Academy of Family Physicians is developing a clinical practice guideline based on the AHRQ review. Current guidelines from the U.S. Department of Veterans Affairs/U.S. Department of Defense and the Global Initiative for Chronic Obstructive Lung Disease recommend a five- to seven-day course of antibiotics and systemic corticosteroids with titrating oxygen therapy to maintain a saturation of 88% to 92% for adults with COPD exacerbations.2,3
Editor's Note:AFP SOR ratings are different from the AHRQ Strength of Evidence ratings. Dr. Saguil is a contributing editor for AFP.
The views expressed in this article are the author's and do not reflect the official policy or position of the Uniformed Services University of the Health Sciences, the Department of Defense, or the U.S. government.
