
Am Fam Physician. 2020;101(9):569-571
Author disclosure: No relevant financial affiliations.
Key Points for Practice
• No medications have been shown to be more effective than placebo for preventing migraine in children and adolescents.
• CBT appears to be effective for reducing migraine frequency in children and adolescents.
• First-line acute migraine treatment in children is ibuprofen; adolescents may benefit from sumatriptan/naproxen tablets, zolmitriptan nasal spray, sumatriptan nasal spray, rizatriptan, or almotriptan.
• Overuse of acute medication to treat migraines increases headache frequency.
From the AFP Editors
Updated recommendations for the prevention and treatment of migraines in children and adolescents were recently published by the American Academy of Neurology and the American Headache Society, and they are based on a systematic review of new evidence published between January 2003 and August 2017. Migraines are common, with a prevalence of 1% to 3% in children three to seven years of age, 4% to 11% in children seven to 11 years of age, and 8% to 23% in those 15 years of age. Prevention should be considered if headaches occur frequently, are severe, and result in significant disability. Migraine-related disability is determined using an evaluation tool such as the Pediatric Migraine Disability Assessment, a six-question scale that measures migraine impact over three months (Table 11,2).
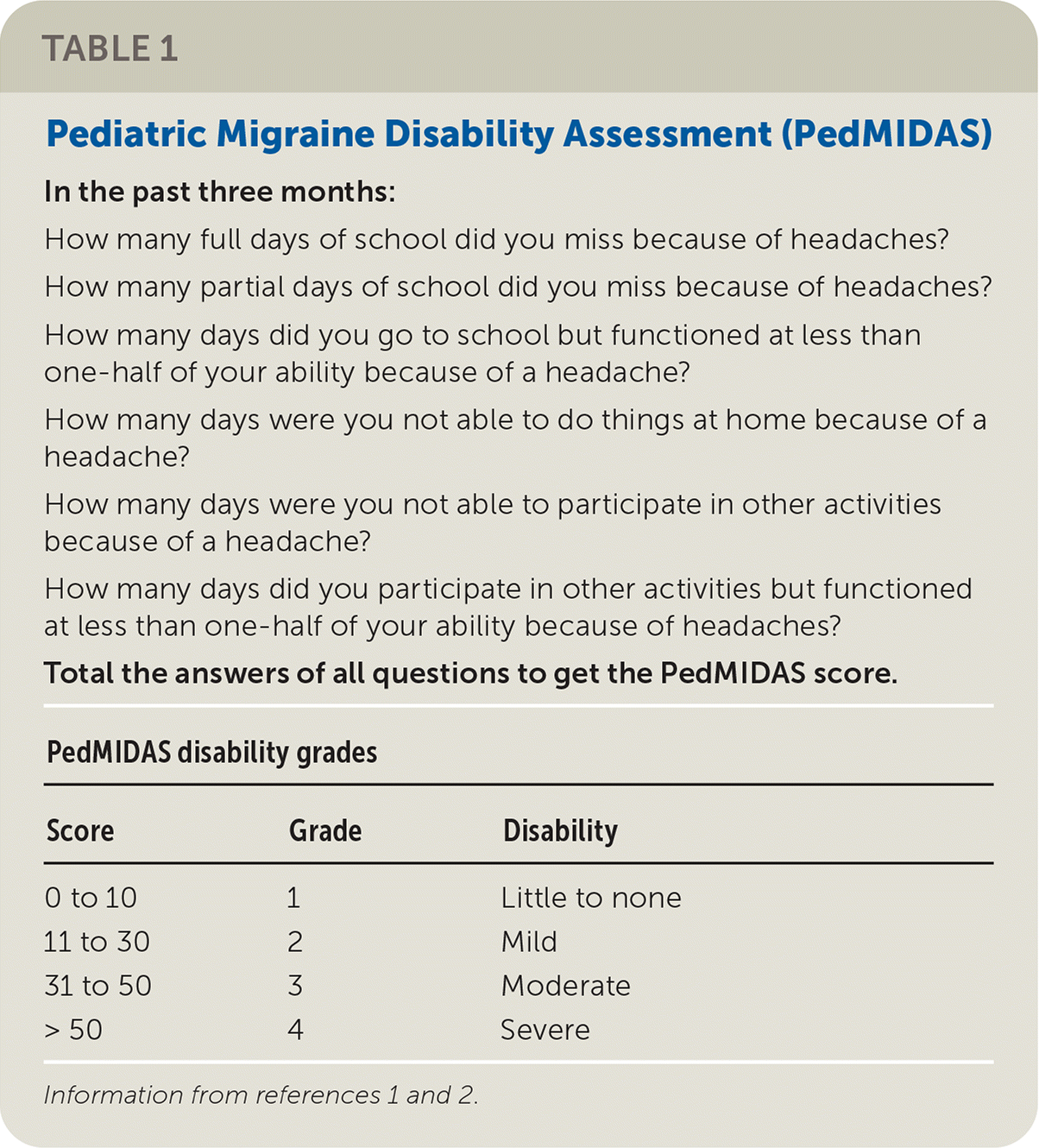
| In the past three months: How many full days of school did you miss because of headaches? How many partial days of school did you miss because of headaches? How many days did you go to school but functioned at less than one-half of your ability because of a headache? How many days were you not able to do things at home because of a headache? How many days were you not able to participate in other activities because of a headache? How many days did you participate in other activities but functioned at less than one-half of your ability because of headaches? | ||
| Total the answers of all questions to get the PedMIDAS score. | ||
| PedMIDAS disability grades | ||
| ScoreGradeDisability | ||
| 0 to 10 | 1 | Little to none |
| 11 to 30 | 2 | Mild |
| 31 to 50 | 3 | Moderate |
| > 50 | 4 | Severe |
Pharmacologic Preventive Treatment
Although the primary outcome in studies of migraine prevention is the percentage of children who achieve a 50% reduction in headache frequency, other important outcomes include a general reduction in frequency, the number of headache days, headache severity, and disability.
TOPIRAMATE (TOPAMAX)
Children and adolescents treated with topiramate may have a decrease in migraine frequency or headache days compared with placebo, but they are as likely as children who receive placebo to have a 50% reduction in headache frequency or a decrease in migraine-related disability.
OTHER PREVENTIVE MEDICATIONS
Extended-release divalproex (Depakote), amitriptyline, and propranolol lack evidence that they are more effective than placebo at reducing headache frequency or disability in children.
ONABOTULINUMTOXINA (BOTOX)
OnabotulinumtoxinA injections are more effective than placebo in adults with chronic migraine (i.e., more than 15 episodes per month) but have shown no benefit in adolescents.
Pharmacologic Preventive Treatment Plus Cognitive Behavior Therapy (CBT)
AMITRIPTYLINE WITH CBT
Children and adolescents 10 to 17 years of age with chronic migraine who are treated with amitriptyline plus CBT are more likely than those receiving amitriptyline and headache education to achieve a 50% reduction in headache frequency and a reduction in headache-related disability.
Prevention Recommendations
Counseling and education are important for patients and their families, especially because of the lack of useful pharmacologic therapy. Behavioral factors that influence the frequency of headaches in adolescents are obesity and overweight, caffeine and alcohol use, lack of physical activity, poor sleeping habits, and tobacco exposure. Losing weight has been shown to reduce headaches in overweight children. Depression is associated with greater headache disability in adolescents.
Consider trigger avoidance for children and adolescents who have frequent headaches or migraine-related disabilities. In adults, more than six headaches per month and overuse of acute medications are associated with increased migraine frequency. Although unproven, overuse of abortive medications is likely associated with more frequent migraines in children and adolescents as well.
Preventive pharmacologic medications were not superior to placebo in most randomized controlled trials, yet the placebo response is high. Up to 61% of children taking placebo had a reduction in headache frequency of 50% or more. Although amitriptyline combined with CBT has been shown to be more effective than amitriptyline without CBT, amitriptyline has a boxed warning from the U.S. Food and Drug Administration concerning risk of suicidal thoughts and behavior in children and adolescents. CBT appears to be the effective intervention.
The teratogenic effects of topiramate and valproate (Depacon) limit their use in young women. Valproate has also been associated with developmental disorders in children. Treatment with more than 200 mg per day of topiramate may decrease the effectiveness of combined oral contraceptives. Folic acid supplementation may decrease congenital malformations in children of women with epilepsy who are taking anticonvulsants and should be considered for young women taking these medications for migraine prevention.
Medication effectiveness and adverse events should be monitored regularly because of the limited evidence for preventive treatment and unclear guidance about when treatment should be stopped.
In children, comorbid negative emotional states such as anxiety, depression, or mental distress do not increase the risk of developing recurrent headache, based on findings of a high-quality systematic review. However, these emotional states increase the risk of headache persistence in patients who experience recurrent headache.
Acute Migraine Treatment
The American Academy of Neurology and American Headache Society conducted a systematic review of the effectiveness of pharmacologic treatment for acute migraine in children and adolescents. There is strong evidence that sumatriptan/naproxen (Treximet) combination oral tablets and zolmitriptan (Zomig) nasal spray relieve pain at two hours more often than placebo. There is moderate evidence that ibuprofen oral solution and sumatriptan (Imitrex) nasal spray relieve pain better than placebo at two hours. Table 2 summarizes the evidence for acute migraine treatments.
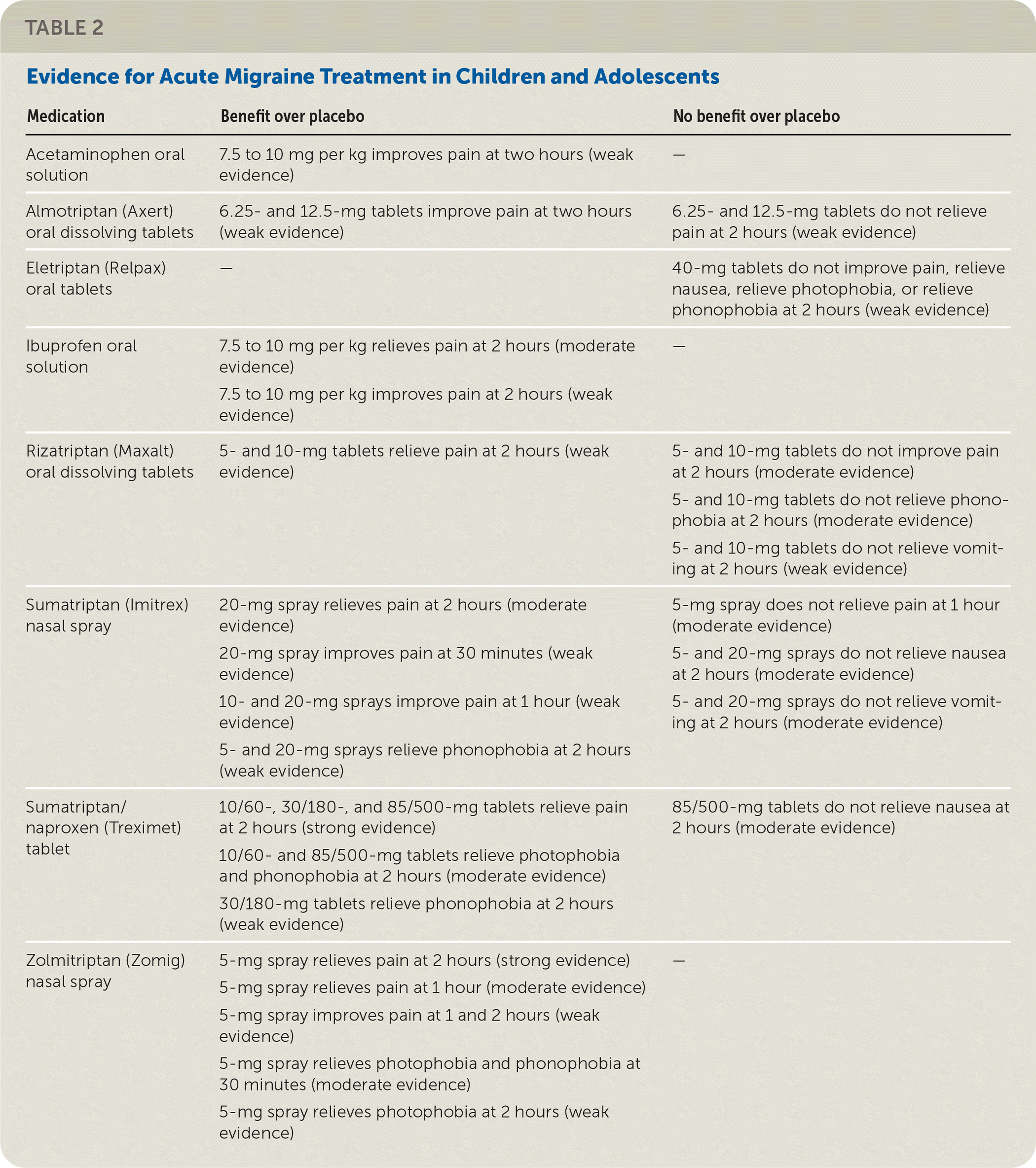
| Medication | Benefit over placebo | No benefit over placebo |
|---|---|---|
| Acetaminophen oral solution | 7.5 to 10 mg per kg improves pain at two hours (weak evidence) | — |
| Almotriptan (Axert) oral dissolving tablets | 6.25- and 12.5-mg tablets improve pain at two hours (weak evidence) | 6.25- and 12.5-mg tablets do not relieve pain at 2 hours (weak evidence) |
| Eletriptan (Relpax) oral tablets | — | 40-mg tablets do not improve pain, relieve nausea, relieve photophobia, or relieve phonophobia at 2 hours (weak evidence) |
| Ibuprofen oral solution | 7.5 to 10 mg per kg relieves pain at 2 hours (moderate evidence) 7.5 to 10 mg per kg improves pain at 2 hours (weak evidence) | — |
| Rizatriptan (Maxalt) oral dissolving tablets | 5- and 10-mg tablets relieve pain at 2 hours (weak evidence) | 5- and 10-mg tablets do not improve pain at 2 hours (moderate evidence) 5- and 10-mg tablets do not relieve phonophobia at 2 hours (moderate evidence) 5- and 10-mg tablets do not relieve vomiting at 2 hours (weak evidence) |
| Sumatriptan (Imitrex) nasal spray | 20-mg spray relieves pain at 2 hours (moderate evidence) 20-mg spray improves pain at 30 minutes (weak evidence) 10- and 20-mg sprays improve pain at 1 hour (weak evidence) 5- and 20-mg sprays relieve phonophobia at 2 hours (weak evidence) | 5-mg spray does not relieve pain at 1 hour (moderate evidence) 5- and 20-mg sprays do not relieve nausea at 2 hours (moderate evidence) 5- and 20-mg sprays do not relieve vomiting at 2 hours (moderate evidence) |
| Sumatriptan/naproxen (Treximet) tablet | 10/60-, 30/180-, and 85/500-mg tablets relieve pain at 2 hours (strong evidence) 10/60- and 85/500-mg tablets relieve photophobia and phonophobia at 2 hours (moderate evidence) 30/180-mg tablets relieve phonophobia at 2 hours (weak evidence) | 85/500-mg tablets do not relieve nausea at 2 hours (moderate evidence) |
| Zolmitriptan (Zomig) nasal spray | 5-mg spray relieves pain at 2 hours (strong evidence) 5-mg spray relieves pain at 1 hour (moderate evidence) 5-mg spray improves pain at 1 and 2 hours (weak evidence) 5-mg spray relieves photophobia and phonophobia at 30 minutes (moderate evidence) 5-mg spray relieves photophobia at 2 hours (weak evidence) | — |
Triptans are prescribed less often in children; therefore, ibuprofen is recommended for initial treatment. In adolescents, sumatriptan/naproxen tablets, sumatriptan nasal spray, and rizatriptan (Maxalt) or almotriptan (Axert) oral dissolving tablets are recommended for initial treatment. Abortive medications are more effective when used early after the onset of migraine in adolescents and adults.
Editor's Note: Migraine preventive treatment is complicated by a significant placebo response, especially in children. In a recent large multicenter trial, the placebo group response rate was 61% for an end point of halving migraine frequency. This response was better than in the two other treatment groups and more than double the placebo effect in adult migraine prevention trials. In children and adolescents, behavioral treatments appear to be more effective for migraine prevention, although the evidence is limited. The American Family Physician Community Blog discusses this in more detail (https://afpjournal.blogspot.com/2019/04/migraine-prevention-whats-changed.html).—Michael J. Arnold, MD, Editorial Fellow
Guideline source: American Academy of Neurology
Evidence rating system used? Yes
Systematic literature search described? Yes
Guideline developed by participants without relevant financial ties to industry? No
Recommendations based on patient-oriented outcomes? Yes
Published source: Neurology. September 10, 2019;93(11):487–509; published correction appears in Neurology. January 7, 2020;94(1):50
Available at: https://n.neurology.org/content/93/11/500.long
