
Am Fam Physician. 2020;102(4):198-199
Original Article: Depression in Children and Adolescents: Evaluation and Treatment
Issue Date: November 15, 2019
See additional reader comments at: https://www.aafp.org/afp/2019/1115/p609.html
To the Editor: We thank Drs. Selph and McDonagh for recommending an evidence-based approach to the evaluation and management of depression in children and adolescents. In this letter, we propose a nuanced approach to medication selection and referral criteria.
We agree that clinicians should consider fluoxetine (Prozac) or escitalopram (Lexapro) as first-line antidepressants for children and adolescents, optimally in conjunction with cognitive behavior therapy or other evidence-based counseling when feasible. Fluoxetine and escitalopram are indeed the only psychotropics approved by the U.S. Food and Drug Administration for the treatment of unipolar depression in children. However, other selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRIs) may be appropriate first- or second-line agents in specific circumstances. Clinicians should consider co-occurring mental health conditions (e.g., sertraline [Zoloft] for treatment of obsessive-compulsive disorder, which may require a high-dose titration), potential drug-drug interactions, adverse effect profiles based on patient factors, and prior response to or preference for a different antidepressant by the patient or family member (which may predict effectiveness and buy-in).1 Furthermore, older adolescents may benefit from a wider range of psychotropics.2
The authors cite a network meta-analysis supporting the use of fluoxetine as the only medication demonstrating effectiveness, but this study assumes heterogeneity in comparisons; pairwise analyses showed that escitalopram and sertraline are also effective.3 Other studies have demonstrated the effectiveness of fluoxetine, escitalopram, sertraline, and venlafaxine,1,3–5 including one that demonstrated clinical improvement with SSRIs or SNRIs over placebo.4 Finally, although refractory cases of depression are often excluded from randomized controlled trials, the TORDIA (Treatment of Resistant Depression in Adolescents) trial showed evidence to support the use of venlafaxine for SSRI-resistant depression.5
When caring for youth with depression, clinicians should optimize school, peer, and community resources to foster a patient's sense of connectedness.1 A patient-clinician relationship with appropriate confidentiality protections and parent involvement should facilitate shared decision-making when developing treatment goals.1 If self-harm or suicidality is identified, clinicians should establish and document an individualized plan. Safety-proofing plans should consider, at a minimum, all medication supplies and accessibility of items that may be used as weapons.
The authors recommend referral if fluoxetine and escitalopram are ineffective. We instead propose that, when trained appropriately, primary care clinicians may take an active role in medication management (Table 11–4) based on experience and patient complexity. We worry that a rigid approach may delay care and cause harm for the most vulnerable youth because of the severe national shortage of child and adolescent mental health specialists.1,6 By applying thoughtfully constructed, evidence-based treatment plans, primary care clinicians can treat youth with depression and fill a critical health services gap.
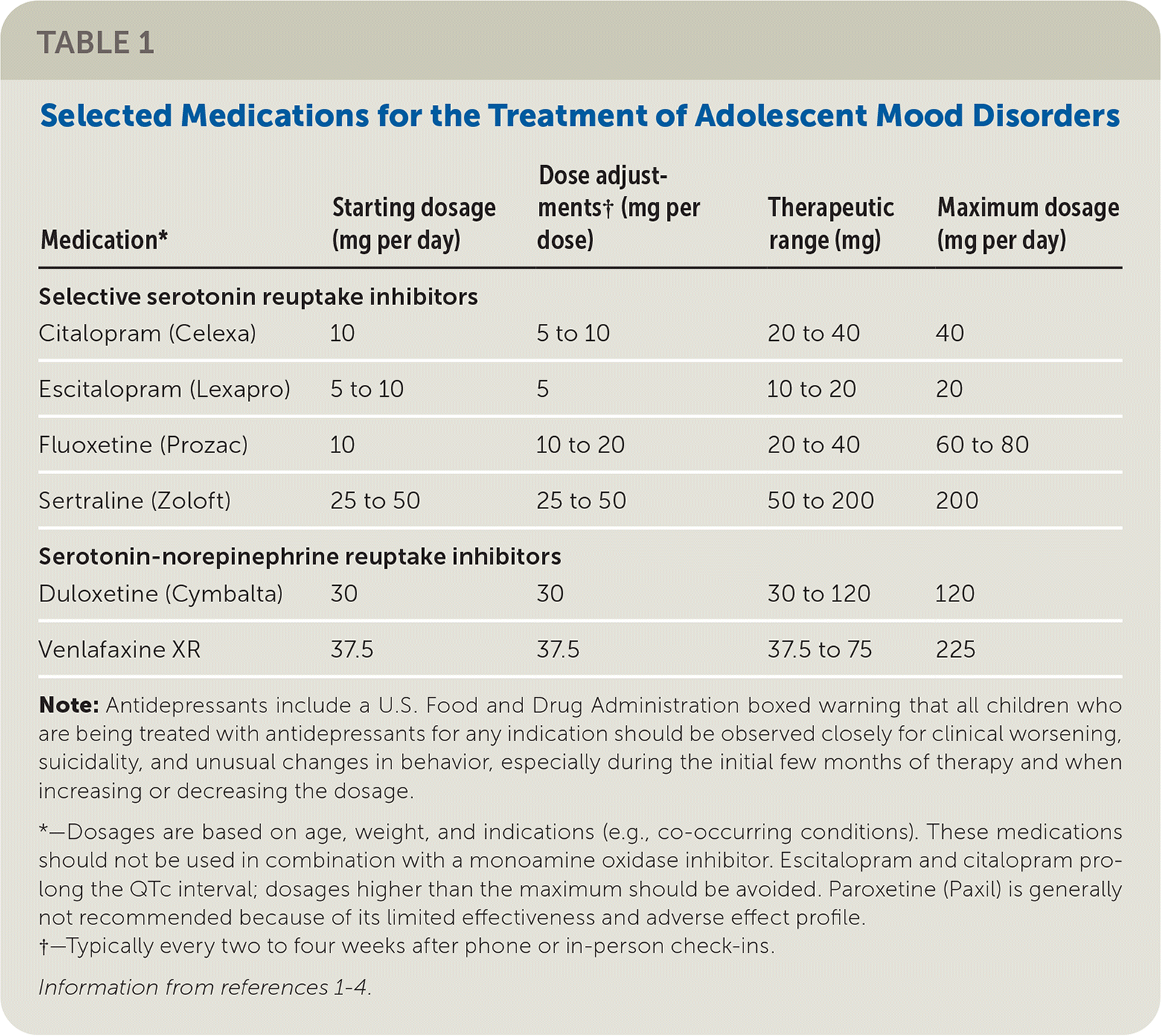
| Medication* | Starting dosage (mg per day) | Dose adjustments† (mg per dose) | Therapeutic range (mg) | Maximum dosage (mg per day) |
|---|---|---|---|---|
| Selective serotonin reuptake inhibitors | ||||
| Citalopram (Celexa) | 10 | 5 to 10 | 20 to 40 | 40 |
| Escitalopram (Lexapro) | 5 to 10 | 5 | 10 to 20 | 20 |
| Fluoxetine (Prozac) | 10 | 10 to 20 | 20 to 40 | 60 to 80 |
| Sertraline (Zoloft) | 25 to 50 | 25 to 50 | 50 to 200 | 200 |
| Serotonin-norepinephrine reuptake inhibitors | ||||
| Duloxetine (Cymbalta) | 30 | 30 | 30 to 120 | 120 |
| Venlafaxine XR | 37.5 | 37.5 | 37.5 to 75 | 225 |
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the U.S. Army Medical Department or the U.S. Army at large.
In Reply: We agree with Drs. Anvari, Carroll, and Klein in recommending a nuanced approach to depression treatment, taking into account patient comorbidities; drug-drug interactions; adverse effect profiles; prior responses or preference for a particular antidepressant; and the availability of school, peer, and community resources. However, treating children and adolescents with complex psychiatric or medical conditions was beyond the scope of our article. Evidence supports the use of fluoxetine and escitalopram as first-line agents for unipolar depression in children and adolescents without complex medical or psychiatric histories.
We believe in basing recommendations on the best evidence available, and the evidence for sertraline and venlafaxine is not as compelling as that for fluoxetine and escitalopram. Evidence showing that sertraline is more effective than placebo is based on two trials that were reported in one publication as if they were one trial. The analysis broke randomization protocols and ignored potential between-trial variability (heterogeneity), which could have inflated the treatment effect.1 The evidence for venlafaxine includes one trial in children and adolescents with major depressive disorder that did not show a significant treatment effect.2 Evidence from the TORDIA trial supports adding cognitive behavior therapy to medication therapy for treatment-resistant depression.3 However, in the trial, switching to venlafaxine was not better than switching to another SSRI, and treatment with an SSRI resulted in fewer adverse effects than treatment with venlafaxine.3
We agree that some primary care physicians may feel comfortable prescribing antidepressants that are not approved for use in children and adolescents. However, we believe physicians should consult with or refer patients to mental health specialists whenever they are uncomfortable with this. We acknowledge the shortage of child and adolescent mental health specialists and that referral may not be easy (e.g., using telehealth vs. an in-person visit4). But at a minimum, phone consultation with a mental health specialist should occur whenever the primary care physician lacks the comfort and expertise needed to appropriately treat patients when first-line therapies have not been successful.
We agree that safety plans for at-risk children and adolescents should include limiting access to traditional weapons, as well as household items that could be used to inflict harm (e.g., kitchen knives, prescription medications).
