
Am Fam Physician. 2020;102(4):250-252
Author disclosure: No relevant financial affiliations.
Key Points for Practice
• Screening reduces CRC mortality in patients 50 to 75 years of age, with greatest benefit in patients older than 60 years.
• The ACP recommends screening average-risk patients with one of the following: colonoscopy every 10 years, flexible sigmoidoscopy every 10 years with biennial FIT, biennial guaiac FOBT, or biennial FIT.
• Because of limited evidence of benefit and increased harms, neither FIT with stool DNA testing nor CT colonography is a recommended screening method.
From the AFP Editors
Colorectal cancer (CRC) is the second leading cause of cancer death in the United States. Up to 80% of CRC develops from colonic adenomas over five to 20 years, illustrating the potential benefit of early detection. Only 10% of adenomas progress to CRC over 10 years; the rest stabilize or regress. The American College of Physicians (ACP) developed a consensus statement for CRC screening of average-risk adults based on their review of six independent guidelines and supporting evidence. People at elevated risk are subject to other screening recommendations and are not addressed here.
Overall Recommendations
The ACP recommends routine screening of average-risk adults between 50 and 75 years of age to reduce CRC mortality. Screening is not recommended after 75 years of age or when life expectancy is less than 10 years. Several screening methods are recommended (Table 1), with decisions based on patient preferences.
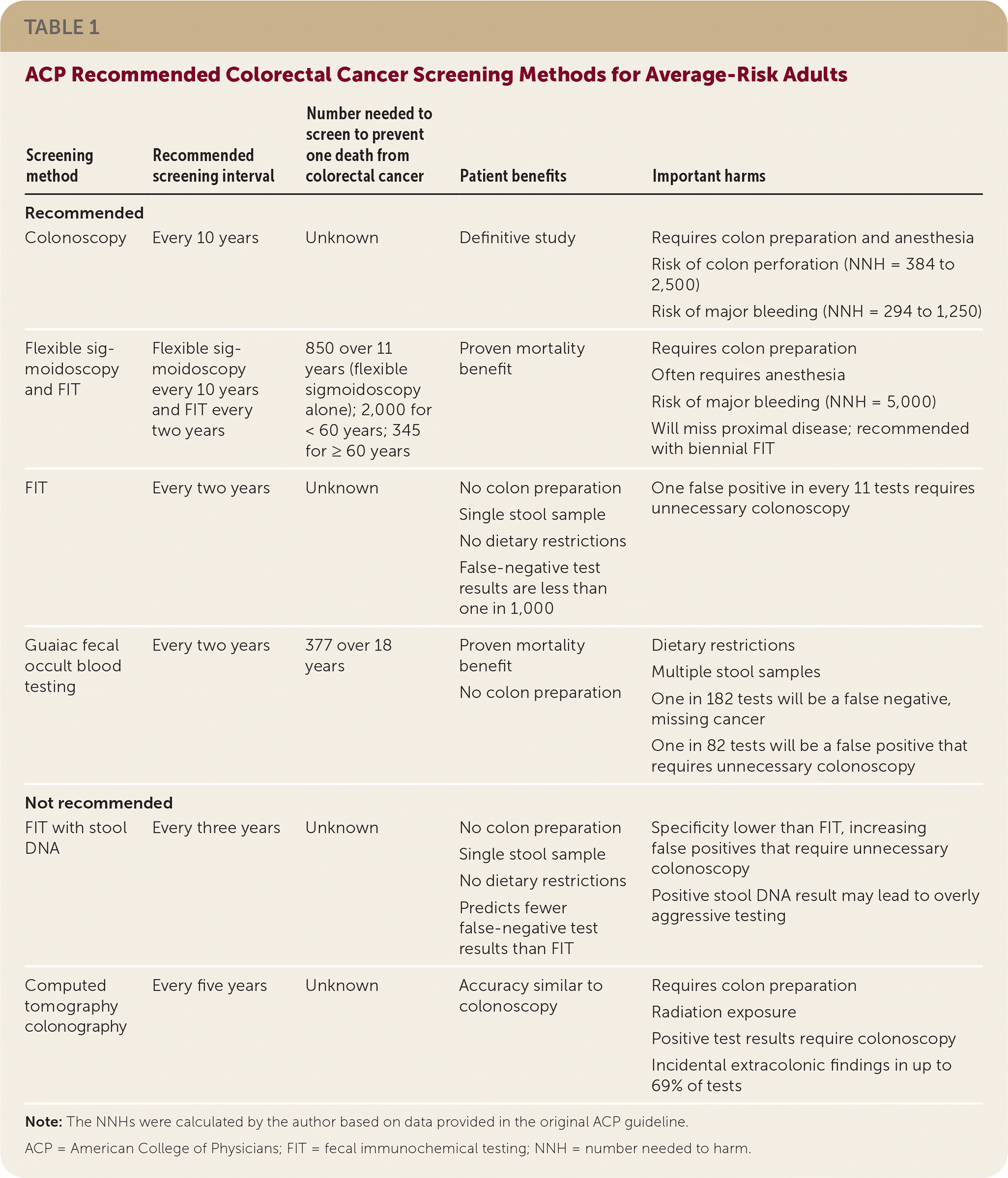
| Screening method | Recommended screening interval | Number needed to screen to prevent one death from colorectal cancer | Patient benefits | Important harms |
|---|---|---|---|---|
| Recommended | ||||
| Colonoscopy | Every 10 years | Unknown | Definitive study | Requires colon preparation and anesthesia Risk of colon perforation (NNH = 384 to 2,500) Risk of major bleeding (NNH = 294 to 1,250) |
| Flexible sigmoidoscopy and FIT | Flexible sigmoidoscopy every 10 years and FIT every two years | 850 over 11 years (flexible sigmoidoscopy alone); 2,000 for < 60 years; 345 for ≥ 60 years | Proven mortality benefit | Requires colon preparation Often requires anesthesia Risk of major bleeding (NNH = 5,000) Will miss proximal disease; recommended with biennial FIT |
| FIT | Every two years | Unknown | No colon preparation Single stool sample No dietary restrictions False-negative test results are less than one in 1,000 | One false positive in every 11 tests requires unnecessary colonoscopy |
| Guaiac fecal occult blood testing | Every two years | 377 over 18 years | Proven mortality benefit No colon preparation | Dietary restrictions Multiple stool samples One in 182 tests will be a false negative, missing cancer One in 82 tests will be a false positive that requires unnecessary colonoscopy |
| Not recommended | ||||
| FIT with stool DNA | Every three years | Unknown | No colon preparation Single stool sample No dietary restrictions Predicts fewer false-negative test results than FIT | Specificity lower than FIT, increasing false positives that require unnecessary colonoscopy Positive stool DNA result may lead to overly aggressive testing |
| Computed tomography colonography | Every five years | Unknown | Accuracy similar to colonoscopy | Requires colon preparation Radiation exposure Positive test results require colonoscopy Incidental extracolonic findings in up to 69% of tests |
Recommended Screening Tests with Strong Evidence
COLONOSCOPY EVERY 10 YEARS
Colonoscopy is accurate in detecting adenomas but has no evidence of decreasing CRC-related mortality. Colonoscopy detects adenomas measuring 10 mm or larger with 89% to 98% sensitivity and 75% to 93% sensitivity for adenomas measuring at least 6 mm. Mortality benefit from colonoscopy is suggested by modeling studies and the proven benefit of flexible sigmoidoscopy.
Harms of colonoscopy include perforation and major bleeding. Perforation occurs in four to 14 per 10,000 procedures, and major bleeding occurs in eight to 34 per 10,000 procedures. Because other screening methods lead to colonoscopy when test results are positive, harms of all testing include those of colonoscopy.
FLEXIBLE SIGMOIDOSCOPY EVERY 10 YEARS WITH FECAL IMMUNOCHEMICAL TESTING EVERY TWO YEARS
Flexible sigmoidoscopy is one of few screening methods proven to reduce CRC mortality, with a number needed to screen of 850 to prevent one CRC death over 11 years. Benefits of flexible sigmoidoscopy increase with advanced age; the number needed to screen is 344 for people 60 years and older but is 2,000 for people younger than 60. Because flexible sigmoidoscopy reduces CRC mortality only in the distal colon, concurrent use of biennial fecal immunochemical testing (FIT) is recommended to increase identification of pathology outside the distal colon.
The advantage of flexible sigmoidoscopy over colonoscopy is a lower likelihood of harm. Colon perforation has not been reported in studies. Bleeding risk is one-fourth of the risk from colonoscopy, with two major bleeds for every 10,000 flexible sigmoidoscopies.
GUAIAC FECAL OCCULT BLOOD TESTING EVERY TWO YEARS
A simple, noninvasive screening method, guaiac fecal occult blood testing (FOBT) is also proven to reduce CRC mortality. Although the number needed to screen for guaiac FOBT is 377 over 18 years for patients 45 to 80 years of age, the primary benefit occurs in patients 60 years and older. Biennial guaiac FOBT is recommended over annual screening because of similar CRC mortality. For every 1,000 tests, 12 results will be false positives and six will be false negatives.
FECAL IMMUNOCHEMICAL TESTING EVERY TWO YEARS
Another stool-based test, FIT eliminates dietary restrictions and multiple samples required for guaiac FOBT. FIT is more accurate than guaiac FOBT, resulting in fewer missed cancers but causes many more false-positive results. For every 1,000 patients who receive FIT, less than one will have a false-negative result, but 88 will have false-positive results, leading to more unnecessary colonoscopies.
Screening Tests Not Recommended Because of Limited Evidence
FECAL IMMUNOCHEMICAL TESTING WITH STOOL DNA PANEL
Based on a single study, combining stool DNA and FIT moderately increases sensitivity with the cost of reduced specificity. Although evidence is lacking, the reduced specificity should exacerbate the high false-positive FIT rate. Positive stool DNA results may lead to more aggressive surveillance without proven benefit.
COMPUTED TOMOGRAPHY COLONOGRAPHY
Computed tomography (CT) colonography appears to have similar accuracy to colonoscopy, with 67% to 94% sensitivity and 86% to 98% specificity for adenomas measuring 10 mm and larger. Clinical benefits of CT colonography have not been studied. Patients require bowel preparation again for follow-up colonoscopy if results are positive. In addition to radiation exposure risks, harms of CT colonography include extracolonic findings in up to 69% of patients, which commonly require further diagnostic workup.
Editor's Notes: The numbers needed to screen and numbers needed to harm were calculated by the author based on data provided in the original ACP guideline.
CRC screening guidelines vary because of organization priorities and evidence limitations. To create these recommendations, the ACP reviewed cited evidence from the American College of Radiology, the Canadian Task Force on Preventive Health Care, the U.S. Preventive Services Task Force (USPSTF), the American Cancer Society, the Scottish Intercollegiate Guidelines Network, and the U.S. Multi-Society Task Force on Colorectal Cancer. The ACP was not able to perform a meta-analysis but cited the best evidence found. The AAFP endorses the 2016 USPSTF recommendations to screen patients between 50 and 75 years of age with FIT, flexible sigmoidoscopy, or colonoscopy and to consider screening patients between 76 and 85 years of age.—Michael J. Arnold, MD, contributing editor
Guideline source: American College of Physicians
Evidence rating system used? Yes
Systematic literature search described? No
Guideline developed by participants without relevant financial ties to industry? Yes
Recommendations based on patient-oriented outcomes? Yes
Published source: Ann Intern Med. November 5, 2019;171(9):643–654
Available at: https://www.acpjournals.org/doi/10.7326/M19-0642
