Am Fam Physician. 2020;102(5):269-270
Author disclosure: No relevant financial affiliations.
PartoSure is an immunoassay that measures the presence of placental alpha macroglobulin-1 (PAMG-1). PAMG-1 is a protein present in the amniotic cavity throughout pregnancy. Detection of PAMG-1 in the vagina in a patient with unruptured membranes is associated with an increased likelihood of delivery in the next seven days. PartoSure was approved by the U.S. Food and Drug Administration in 2018 for patients with a singleton pregnancy between 24 0/7 and 34 6/7 weeks' gestation, signs and symptoms of preterm labor, and cervical dilatation less than 3 cm.1 The test result is positive when at least 1 ng per mL of PAMG-1 is detected.2
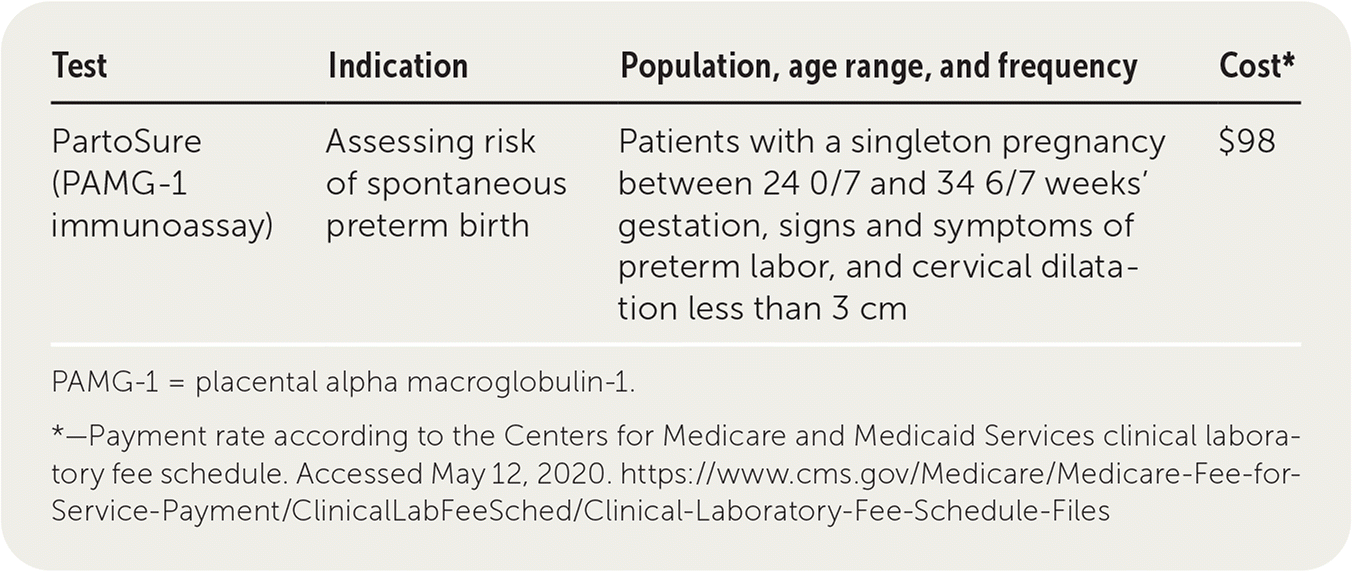
| Test | Indication | Population, age range, and frequency | Cost* |
|---|---|---|---|
| PartoSure (PAMG-1 immunoassay) | Assessing risk of spontaneous preterm birth | Patients with a singleton pregnancy between 24 0/7 and 34 6/7 weeks' gestation, signs and symptoms of preterm labor, and cervical dilatation less than 3 cm | $98 |
Accuracy
A recent systematic review summarized test characteristics of PAMG-1 testing for predicting risk of delivery within seven days in patients with symptoms of preterm labor before 37 weeks' gestation, cervical dilatation of less than 3 cm, and clinically intact membranes.3 The review included 14 prospective or cohort studies with 2,278 total patients. All studies were published between 2015 and 2018. The trials varied in quality, and many met only one or two out of four quality criteria. The review did not list author conflicts of interest or the number of studies that were industry sponsored.
For predicting delivery within seven days, PAMG-1 testing has a sensitivity of 76%, a specificity of 97%, a positive likelihood ratio of 22.51, and a negative likelihood ratio of 0.24. The rate of delivery within seven days varied among the studies from 2.0% to 20.8%. The overall rate of delivery within seven days in the 14 studies was 6.9%.3 By comparison, the systematic review reported that for the same outcome, fetal fibronectin testing has a sensitivity of 58%, a specificity of 84%, a positive likelihood ratio of 3.63, and a negative likelihood ratio of 0.50.3 Table 1 includes posttest probabilities of delivering within seven days given varying pretest probabilities and findings on PAMG-1 or fetal fibronectin testing.3
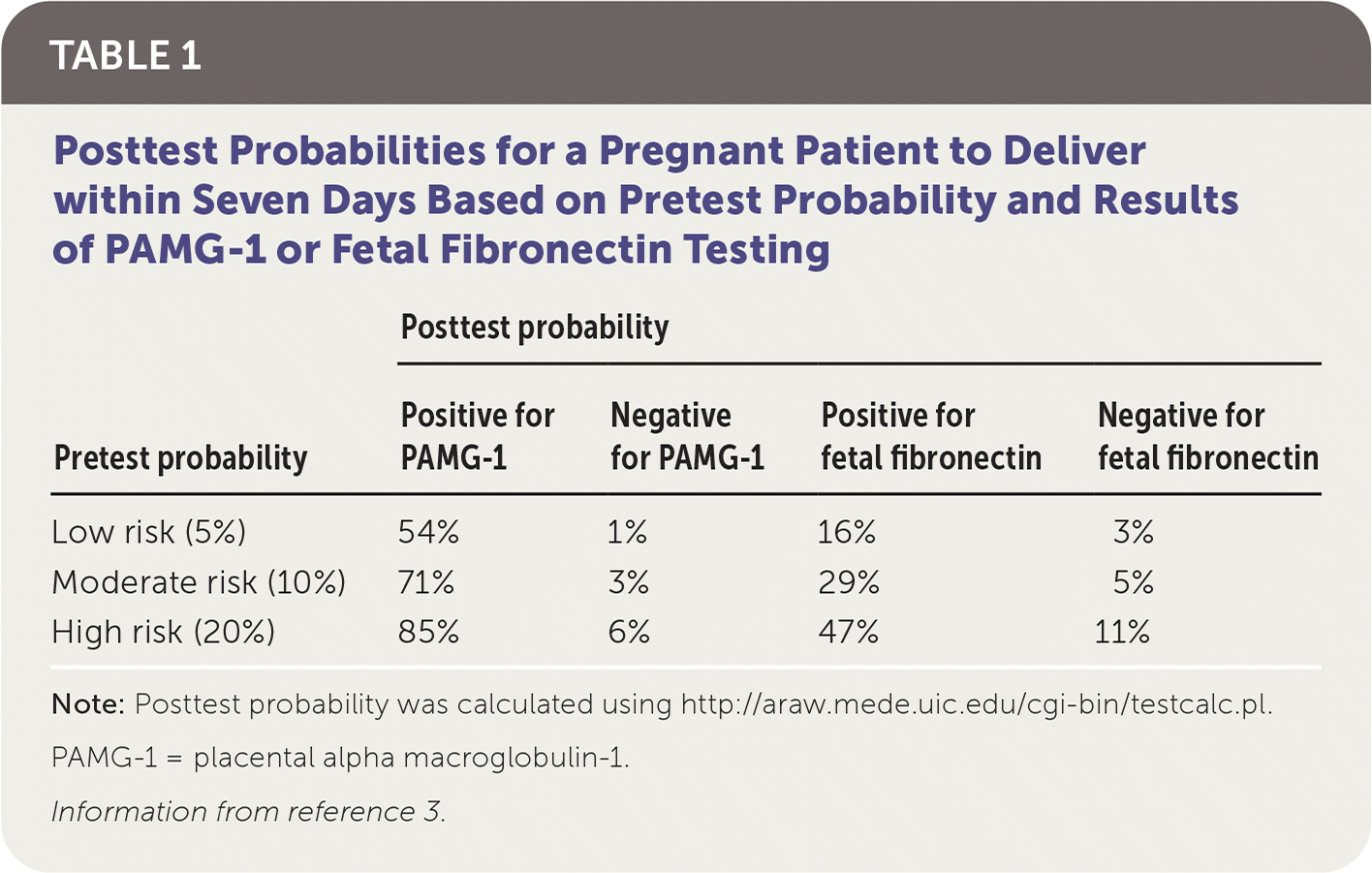
| Pretest probability | Posttest probability | |||
|---|---|---|---|---|
| Positive for PAMG-1 | Negative for PAMG-1 | Positive for fetal fibronectin | Negative for fetal fibronectin | |
| Low risk (5%) | 54% | 1% | 16% | 3% |
| Moderate risk (10%) | 71% | 3% | 29% | 5% |
| High risk (20%) | 85% | 6% | 47% | 11% |
Benefit
The goal of predicting preterm delivery within seven days using a PAMG-1 or fetal fibronectin test is to administer appropriate therapy to improve maternal or neonatal outcomes. A single course of corticosteroids improves neonatal outcomes and is recommended in patients likely to deliver within seven days.5 Magnesium sulfate may also provide neuroprotective benefit in patients delivering before 32 weeks' gestation.6 Tocolysis may allow time to arrange for an appropriate level of care.
There are no published data on the use of PAMG-1 testing to improve maternal or neonatal outcomes. A systematic review of six randomized trials including 546 singleton gestations with threatened preterm labor showed no statistically significant difference in the number of patients who delivered within seven days when comparing those who had fetal fibronectin testing to aid in management and those who did not (12.85% vs. 14.5%; relative risk = 0.76; 95% CI, 0.47 to 1.21).7 A Cochrane review found low-quality evidence that use of fetal fibronectin testing may reduce preterm birth before 37 weeks' gestation (21.6% vs. 29.2% in the control group; relative risk = 0.72; 95% CI, 0.52 to 1.01.).8 It is not known how PAMG-1 compares with fetal fibronectin in improving maternal or neonatal outcomes.
Harms
There are no known harms from performing a PAMG-1 or fetal fibronectin test. PAMG-1 testing requires a vaginal swab, and fetal fibronectin testing requires a cervicovaginal swab using a speculum.9 These procedures could cause some patient discomfort. False negatives could wrongly reassure patients and clinicians. False positives could lead to unnecessary hospitalizations and interventions.
Cost
Medicare reimburses $98 for the PartoSure immunoassay, compared with $64 for the fetal fibronectin test.10
Bottom Line
The use of PAMG-1 testing is limited. The test may be a useful adjunct to determine which patients with threatened preterm labor might benefit from corticosteroid administration to improve neonatal outcomes. PAMG-1 is more sensitive and specific than fetal fibronectin for the prediction of delivery within seven days. However, it is not known whether the use of PAMG-1 testing improves maternal or neonatal outcomes.
Editor's Note: Dr. Brown is a contributing editor for AFP.