
Am Fam Physician. 2020;102(7):online
Author disclosure: No relevant financial affiliations.
Clinical Question
What are the first-line pharmacologic treatment options for alcohol use disorder?
Evidence-Based Answer
Acamprosate and naltrexone should be used as first-line agents for treatment of alcohol use disorder and are effective for reducing relapse rates. Agent selection should be based on comorbid conditions and adherence to the dosing regimen. (Strength of Recommendation [SOR]: A, based on a meta-analysis.) Combining the two agents may provide additional benefit early in treatment. (SOR: B, based on a single randomized controlled trial [RCT]).
Evidence Summary
A 2014 meta-analysis of 22 RCTs and one cohort study (N = 22,803) evaluated relapse rates in patients who received acamprosate or naltrexone, alone or in combination, for at least 12 weeks.1 The primary outcome was a return to alcohol consumption, classified as any or heavy consumption (at least five drinks per day for men or at least four for women). Rates of return to any consumption improved with either agent. The number needed to treat (NNT) for return to any consumption was 12 for acamprosate (95% CI, 8 to 26; 16 trials; n = 4,847) and 20 for naltrexone (95% CI, 11 to 500; 16 trials; n = 2,347). Naltrexone monotherapy demonstrated benefit for heavy consumption (NNT = 12; 95% CI, 8 to 26; 19 trials; n = 2,875).
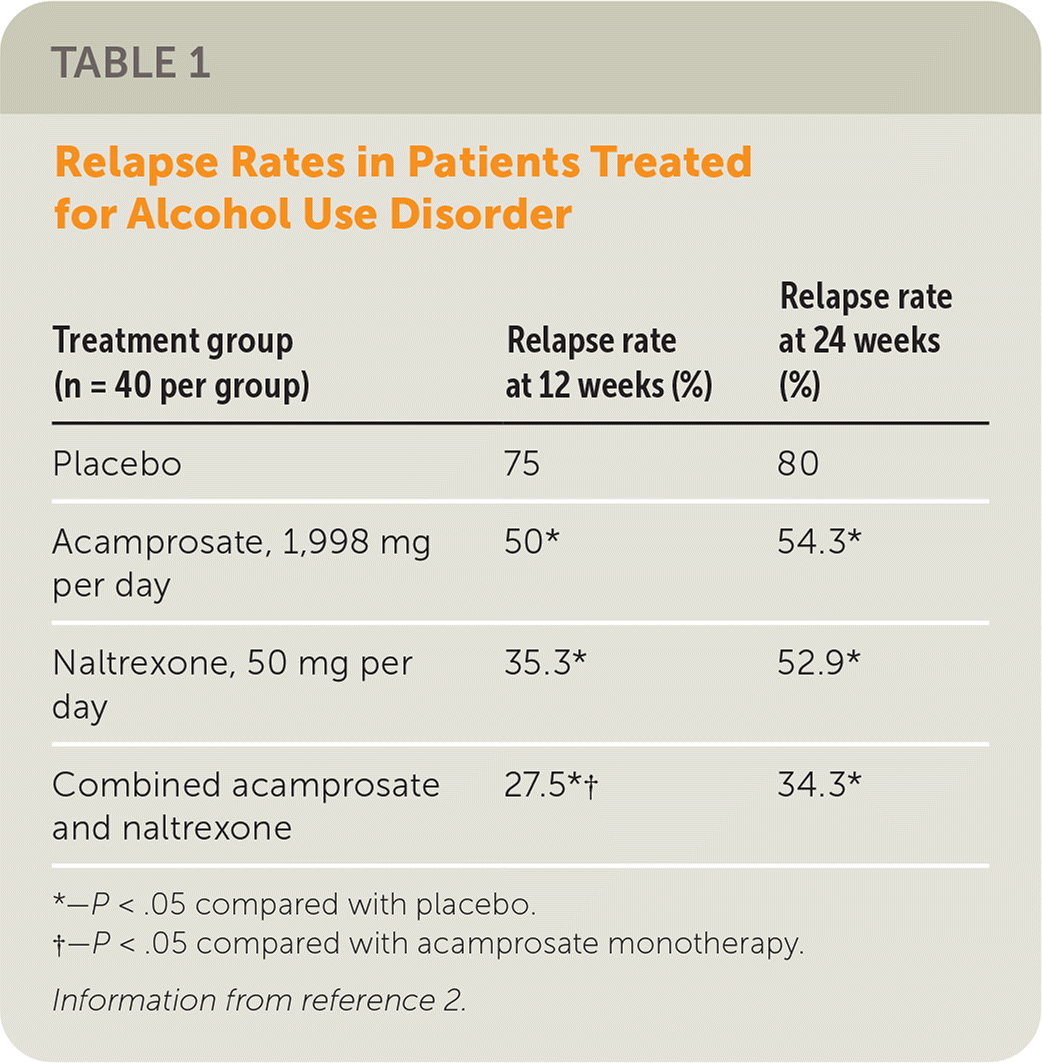
A 2004 RCT examined the effectiveness of naltrexone or acamprosate, alone or in combination, in preventing relapse in newly detoxified adults (N = 160).2 Table 1 shows relapse rates at 12 and 24 weeks among the four treatment groups.2 Acamprosate, naltrexone, and combination therapy were significantly more effective than placebo at 12 and 24 weeks (P < .05). At 12 weeks, the relapse rate among patients receiving combination therapy was significantly lower than in the acamprosate group (P < .05), but this significance was not observed at 24 weeks. There was an increase in nausea and diarrhea in the combination therapy group (P < .05).
Recommendations from Others
The American Psychiatric Association (APA) and the National Institute for Health and Care Excellence recommend naltrexone and acamprosate as the preferred pharmacologic options for patients with alcohol use disorder, in combination with cognitive behavioral interventions.3,4 The APA recommends against acamprosate therapy in patients with severe renal impairment, and against naltrexone in those with hepatic failure or acute hepatitis.3 Acamprosate is typically taken three times daily; naltrexone is taken once daily and is also available in a long-acting parenteral formulation.
Copyright © Family Physicians Inquiries Network. Used with permission.
