
Am Fam Physician. 2020;102(9):558-561
Author disclosure: No relevant financial affiliations.
Key Clinical Issue
What are the benefits and harms of nonpharmacologic and pharmacologic treatments for depressive disorders in children and adolescents?
Evidence-Based Answer
Cognitive behavior therapy (CBT), family therapy, exercise, and spirituality reduce depressive symptoms in adolescents with no evidence of harms. (Strength of Recommendation [SOR]: B, based on inconsistent or limited-quality patient-oriented evidence.) Selective serotonin reuptake inhibitors (SSRIs) improve depressive symptoms and response in adolescents with major depressive disorder. (SOR: B, based on inconsistent or limited-quality patient-oriented evidence.) Serious adverse events and withdrawal because of adverse events are more common with SSRIs compared with placebo. (SOR: B, based on inconsistent or limited-quality patient-oriented evidence.) Paroxetine may cause increased suicidal ideation or behavior in adolescents and children. (SOR: B, based on inconsistent or limited-quality patient-oriented evidence.) There is no symptom improvement with serotonin-norepinephrine reuptake inhibitors compared with placebo in adolescents with major depressive disorder. (SOR: B, based on inconsistent or limited-quality patient-oriented evidence.) CBT combined with fluoxetine improves depressive symptoms, remission, and functional status more than CBT alone.1 (SOR: B, based on inconsistent or limited-quality patient-oriented evidence.)
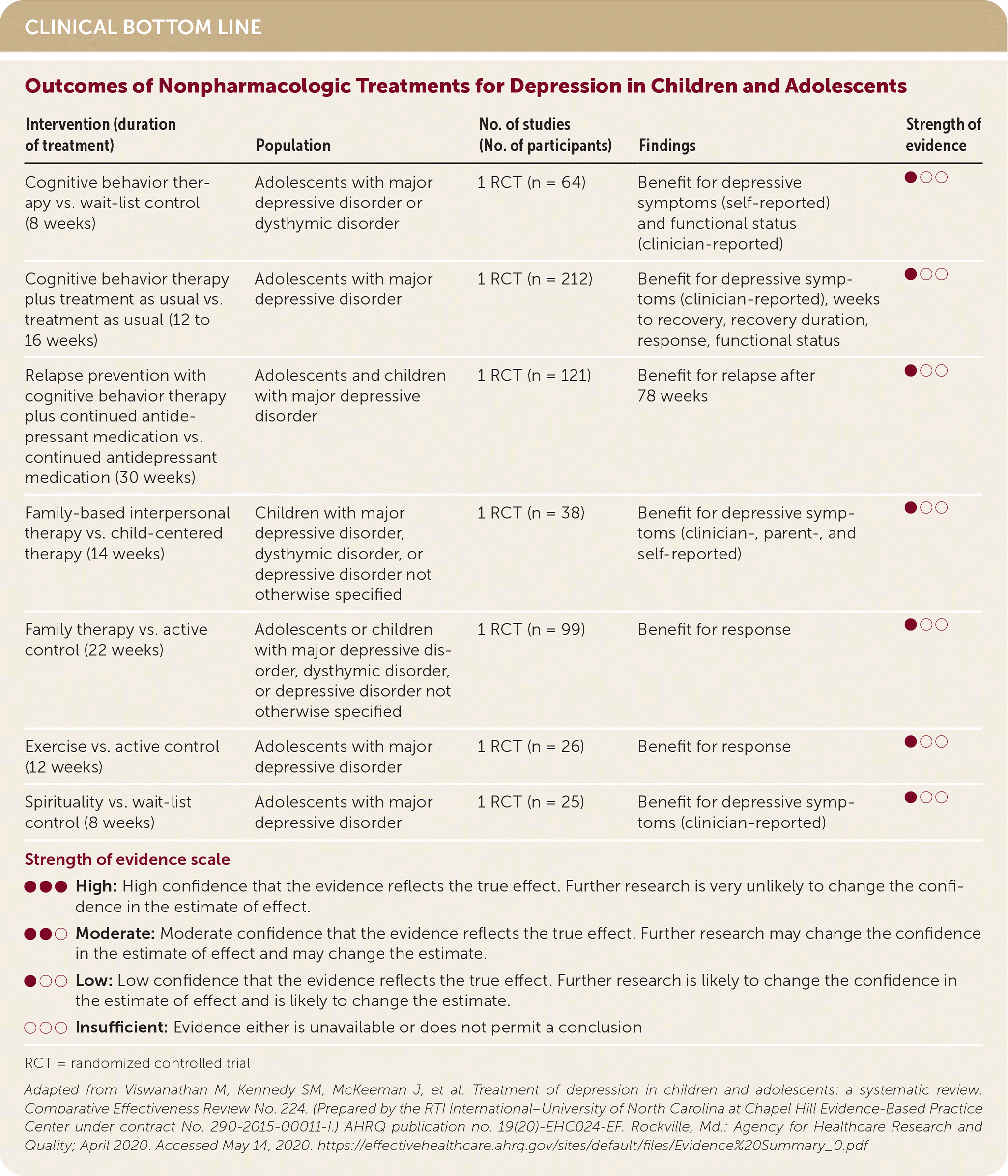
| Intervention (duration of treatment) | Population | No. of studies (No. of participants) | Findings | Strength of evidence |
|---|---|---|---|---|
| Cognitive behavior therapy vs. wait-list control (8 weeks) | Adolescents with major depressive disorder or dysthymic disorder | 1 RCT (n = 64) | Benefit for depressive symptoms (self-reported) and functional status (clinician-reported) | ●○○ |
| Cognitive behavior therapy plus treatment as usual vs. treatment as usual (12 to 16 weeks) | Adolescents with major depressive disorder | 1 RCT (n = 212) | Benefit for depressive symptoms (clinician-reported), weeks to recovery, recovery duration, response, functional status | ●○○ |
| Relapse prevention with cognitive behavior therapy plus continued antidepressant medication vs. continued antidepressant medication (30 weeks) | Adolescents and children with major depressive disorder | 1 RCT (n = 121) | Benefit for relapse after 78 weeks | ●○○ |
| Family-based interpersonal therapy vs. child-centered therapy (14 weeks) | Children with major depressive disorder, dysthymic disorder, or depressive disorder not otherwise specified | 1 RCT (n = 38) | Benefit for depressive symptoms (clinician-, parent-, and self-reported) | ●○○ |
| Family therapy vs. active control (22 weeks) | Adolescents or children with major depressive disorder, dysthymic disorder, or depressive disorder not otherwise specified | 1 RCT (n = 99) | Benefit for response | ●○○ |
| Exercise vs. active control (12 weeks) | Adolescents with major depressive disorder | 1 RCT (n = 26) | Benefit for response | ●○○ |
| Spirituality vs. wait-list control (8 weeks) | Adolescents with major depressive disorder | 1 RCT (n = 25) | Benefit for depressive symptoms (clinician-reported) | ●○○ |
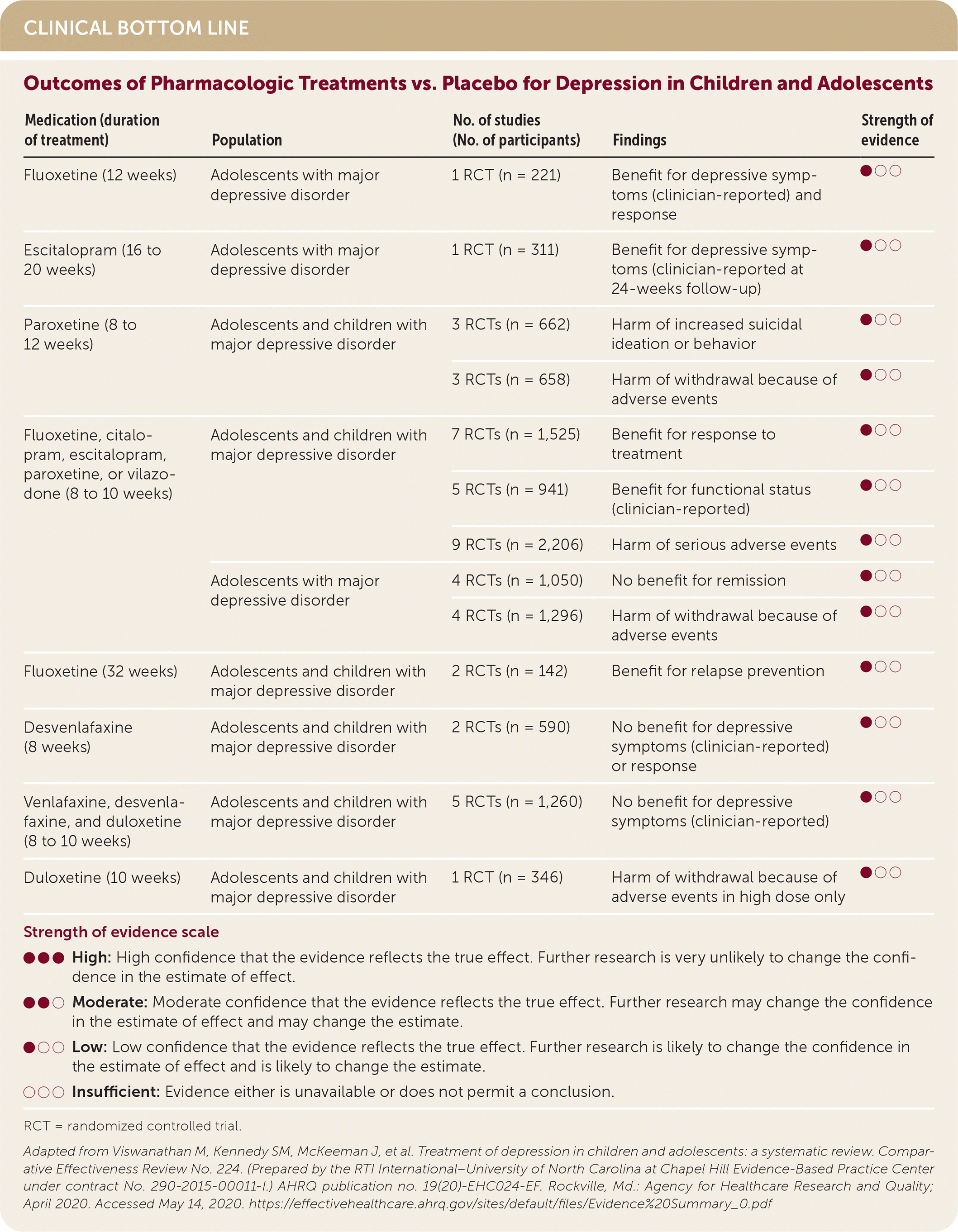
| Medication (duration of treatment) | Population | No. of studies (No. of participants) | Findings | Strength of evidence |
|---|---|---|---|---|
| Fluoxetine (12 weeks) | Adolescents with major depressive disorder | 1 RCT (n = 221) | Benefit for depressive symptoms (clinician-reported) and response | ●○○ |
| Escitalopram (16 to 20 weeks) | Adolescents with major depressive disorder | 1 RCT (n = 311) | Benefit for depressive symptoms (clinician-reported at 24-weeks follow-up) | ●○○ |
| Paroxetine (8 to 12 weeks) | Adolescents and children with major depressive disorder | 3 RCTs (n = 662) | Harm of increased suicidal ideation or behavior | ●○○ |
| 3 RCTs (n = 658) | Harm of withdrawal because of adverse events | ●○○ | ||
| Fluoxetine, citalopram, escitalopram, paroxetine, or vilazodone (8 to 10 weeks) | Adolescents and children with major depressive disorder | 7 RCTs (n = 1,525) | Benefit for response to treatment | ●○○ |
| 5 RCTs (n = 941) | Benefit for functional status (clinician-reported) | ●○○ | ||
| 9 RCTs (n = 2,206) | Harm of serious adverse events | ●○○ | ||
| Adolescents with major depressive disorder | 4 RCTs (n = 1,050) | No benefit for remission | ●○○ | |
| 4 RCTs (n = 1,296) | Harm of withdrawal because of adverse events | ●○○ | ||
| Fluoxetine (32 weeks) | Adolescents and children with major depressive disorder | 2 RCTs (n = 142) | Benefit for relapse prevention | ●○○ |
| Desvenlafaxine (8 weeks) | Adolescents and children with major depressive disorder | 2 RCTs (n = 590) | No benefit for depressive symptoms (clinician-reported) or response | ●○○ |
| Venlafaxine, desvenlafaxine, and duloxetine (8 to 10 weeks) | Adolescents and children with major depressive disorder | 5 RCTs (n = 1,260) | No benefit for depressive symptoms (clinician-reported) | ●○○ |
| Duloxetine (10 weeks) | Adolescents and children with major depressive disorder | 1 RCT (n = 346) | Harm of withdrawal because of adverse events in high dose only | ●○○ |
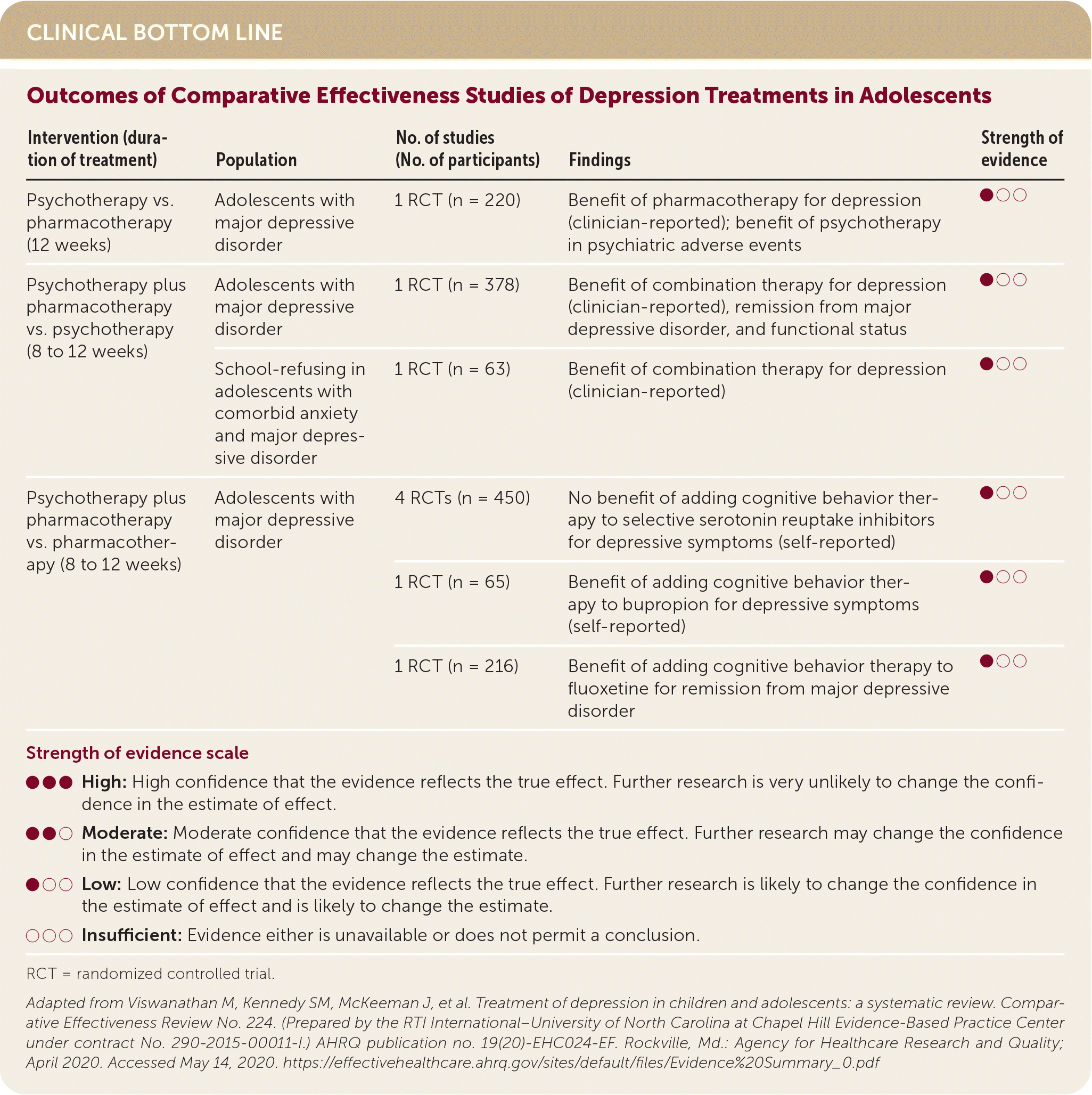
| Intervention (duration of treatment) | Population | No. of studies (No. of participants) | Findings | Strength of evidence |
|---|---|---|---|---|
| Psychotherapy vs. pharmacotherapy (12 weeks) | Adolescents with major depressive disorder | 1 RCT (n = 220) | Benefit of pharmacotherapy for depression (clinician-reported); benefit of psychotherapy in psychiatric adverse events | ●○○ |
| Psychotherapy plus pharmacotherapy vs. psychotherapy (8 to 12 weeks) | Adolescents with major depressive disorder | 1 RCT (n = 378) | Benefit of combination therapy for depression (clinician-reported), remission from major depressive disorder, and functional status | ●○○ |
| School-refusing in adolescents with comorbid anxiety and major depressive disorder | 1 RCT (n = 63) | Benefit of combination therapy for depression (clinician-reported) | ●○○ | |
| Psychotherapy plus pharmacotherapy vs. pharmacotherapy (8 to 12 weeks) | Adolescents with major depressive disorder | 4 RCTs (n = 450) | No benefit of adding cognitive behavior therapy to selective serotonin reuptake inhibitors for depressive symptoms (self-reported) | ●○○ |
| 1 RCT (n = 65) | Benefit of adding cognitive behavior therapy to bupropion for depressive symptoms (self-reported) | ●○○ | ||
| 1 RCT (n = 216) | Benefit of adding cognitive behavior therapy to fluoxetine for remission from major depressive disorder | ●○○ |
Practice Pointers
Nearly one in five adolescents has a depressive disorder at some point before adulthood.2 Primary care physicians cite many barriers to treating depression in adolescents and children. One of those barriers is a concern about increased suicidality after the U.S. Food and Drug Administration placed a boxed warning on antidepressants for children and adolescents in 2004 and expanded that warning in 2018.3,4
This Agency for Healthcare Research and Quality (AHRQ) review assessed the benefits and harms of nonpharmacologic and pharmacologic treatment of depressive disorders in children and adolescents. The review identified 60 relevant studies (including 43 in the United States) that treated participants for at least six weeks and reported both benefits and harms.1
The AHRQ review found limited evidence of benefit for depressive symptoms from CBT, family therapy, exercise, and spirituality, although none of these treatments showed evidence of harm. Family therapy focuses on the family and interpersonal relationships, as opposed to other forms of therapy, which are child focused.5,6 The spirituality intervention was an eight-week online program based on principles of spirituality such as gratitude and forgiveness.7 When pharmacotherapy was added to nonpharmacologic therapy, there was a benefit for symptoms and functional status compared with nonpharmacologic therapies alone.1
Depressive symptoms and function were improved with SSRIs but not serotoninnorepinephrine reuptake inhibitors. Patients taking SSRIs were more likely to withdraw from treatment because of serious adverse events. Paroxetine may be associated with increased suicidal ideation. This was evident only when all studies were included. When the analysis excluded two studies with a high risk of bias, there was insufficient evidence to suggest an increased risk of suicidal ideation. When CBT was added to fluoxetine, depressive symptoms and remission improved compared with fluoxetine alone.1
Family physicians play a critical role in identifying and treating depressive disorders in children and adolescents. Physicians following the U.S. Preventive Services Task Force recommendation to screen all adolescents from 12 to 18 years of age for major depressive disorder may feel less comfortable managing treatment.8 To support physicians, the American Academy of Pediatrics has published guidelines for treating adolescent depression in primary care.2 These guidelines recommend considering active support and monitoring for six to eight weeks before beginning treatment and monitoring patients monthly for up to two years after remission because symptom recurrence is common. The guidelines emphasize evidence-based treatments such as CBT and SSRIs.2
Physicians may consider initiating SSRIs for children and adolescents with major depressive disorder. Only fluoxetine and escitalopram are approved by the U.S. Food and Drug Administration for the treatment of depression in this age group.9 Options such as CBT or family therapy with or without SSRIs should be considered. Exercise and spirituality may also be incorporated into the treatment of adolescents with depression because they improve symptoms with no known harms.1
Editor's Note: American Family Physician SOR ratings are different from the AHRQ Strength of Evidence ratings.
