
Am Fam Physician. 2020;102(9):550-557
Patient information: See related handout on genitourinary syndrome of menopause, written by the authors of this article.
Author disclosure: No relevant financial affiliations.
Common benign chronic vulvar conditions include genitourinary syndrome of menopause (formerly called vulvovaginal atrophy), lichen sclerosus, lichen planus, lichen simplex chronicus, and vulvodynia. Genitourinary syndrome of menopause results from the hypoestrogenic state that leads to atrophy of normal vulvar and vaginal tissues. It is typically treated with lubricants, moisturizers, and intravaginal estrogen. Lichen sclerosus is an inflammatory condition characterized by intense vulvar itching. It is treated with topical steroids or, in some cases, topical calcineurin inhibitors. Patients with lichen sclerosus are at risk of vulvar squamous cell carcinoma and should be monitored closely for malignancy. Lichen planus is an inflammatory autoimmune disorder that can affect the vulva and vagina in addition to other skin and mucosal surfaces. The first-line treatment is topical steroids, and significant scarring can occur if left untreated. Lichen simplex chronicus manifests as persistent itching and scratching of the vulvar skin that leads to thickened epithelium. Breaking the itch-scratch cycle, often with topical steroids, is the key to treatment. Vulvodynia is a common vulvar pain disorder and is a diagnosis of exclusion. A multimodal treatment approach typically includes vulvar hygiene, physical therapy, psychosocial interventions, and antineuropathy medications.
Genitourinary Syndrome of Menopause
Genitourinary syndrome of menopause (GSM), formerly called vulvovaginal atrophy, is a constellation of symptoms associated with changes in vulvar and lower urinary tract anatomy due to menopause-related decreases in levels of estrogen and other hormones. Vaginal dryness and other GSM symptoms are thought to occur in approximately 50% of individuals within three years after the onset of menopause.3
PRESENTATION
Symptoms of GSM include vaginal or vulvar dryness, burning, itching, dyspareunia, bleeding, vaginal discharge, urinary urgency, and recurrent urinary tract infections. These symptoms are not often reported to physicians,4 so proactive questioning should be used to elicit whether they are present (e.g., “Do you have any questions about sex you would like to discuss today?” “Have you noticed any symptoms like vaginal pain or burning recently?”).
Although patients with GSM are by definition postmenopausal, anyone experiencing a hypoestrogenic state may have symptoms of GSM, including those who are breastfeeding, who have central (hypothalamic) amenorrhea, or who are taking medications with antiestrogenic effects (e.g., raloxifene [Evista], tamoxifen, and other selective estrogen receptor modulators; gonadotropin-releasing hormone agonists; aromatase inhibitors).
DIAGNOSIS
On examination, the vulvar epithelium of patients with GSM is typically thin, pale, and/or erythematous. Other signs include loss of vaginal rugae, introital narrowing, decreased tissue elasticity, urethral caruncle, and mucosal prolapse; resorption of the labia minora also may be apparent5 (Figure 1). Diagnosis can be made clinically based on physical examination findings alone. If litmus paper is available, the vaginal pH in untreated postmenopausal patients will be greater than 4.5. A biopsy is not required.
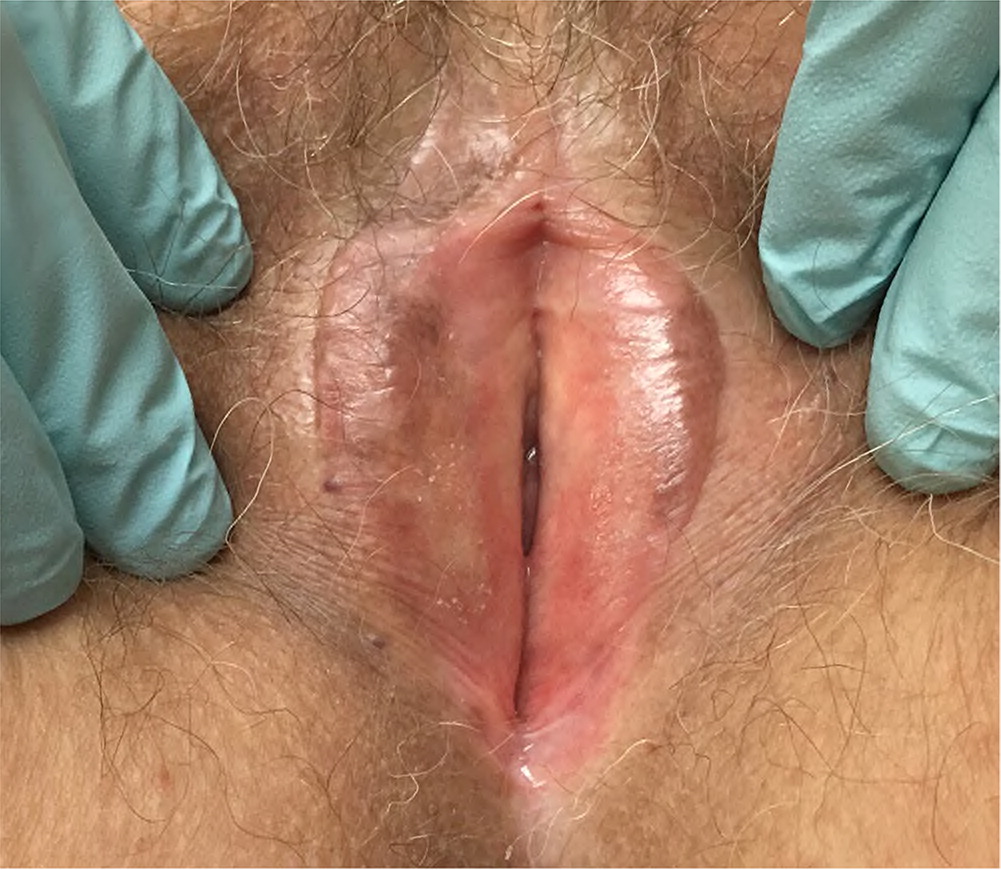
TREATMENT
First-line treatment for GSM involves avoidance of irritants, the use of vaginal moisturizers one to three times per week, and the use of vaginal lubricants during intercourse. However, these measures are often insufficient to resolve symptoms. If further treatment is needed, low-dose intravaginal estrogen therapy is preferred.6–8 Vaginal estrogen is available in the form of creams, vaginal tablets, or a vaginal ring; all are acceptable for the treatment of GSM8 (Table 1). Local estrogen administration is safe, effective, and proven to improve symptoms of atrophy and to prevent development of recurrent urinary tract infections.6,7
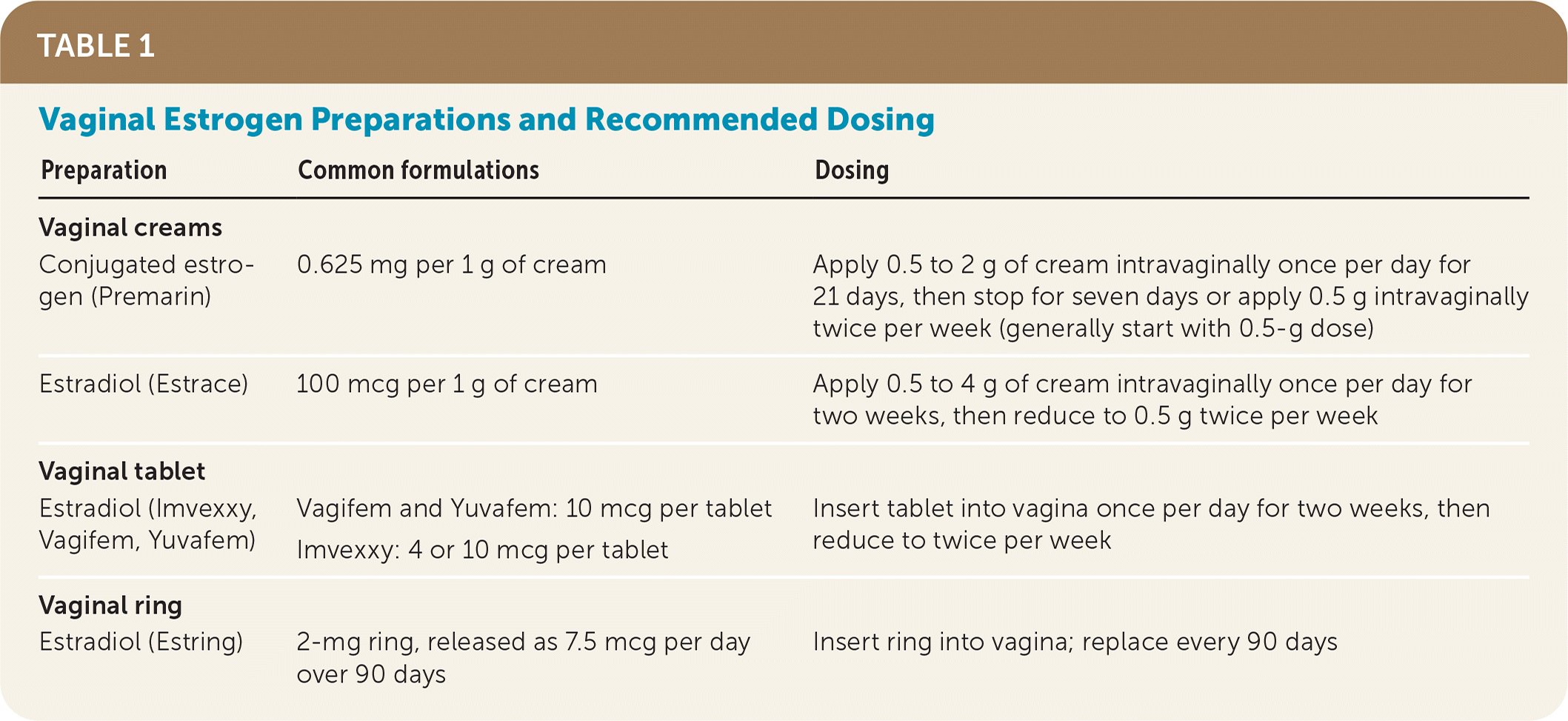
| Preparation | Common formulations | Dosing |
|---|---|---|
| Vaginal creams | ||
| Conjugated estrogen (Premarin) | 0.625 mg per 1 g of cream | Apply 0.5 to 2 g of cream intravaginally once per day for 21 days, then stop for seven days or apply 0.5 g intravaginally twice per week (generally start with 0.5-g dose) |
| Estradiol (Estrace) | 100 mcg per 1 g of cream | Apply 0.5 to 4 g of cream intravaginally once per day for two weeks, then reduce to 0.5 g twice per week |
| Vaginal tablet | ||
| Estradiol (Imvexxy, Vagifem, Yuvafem) | Vagifem and Yuvafem: 10 mcg per tablet Imvexxy: 4 or 10 mcg per tablet | Insert tablet into vagina once per day for two weeks, then reduce to twice per week |
| Vaginal ring | ||
| Estradiol (Estring) | 2-mg ring, released as 7.5 mcg per day over 90 days | Insert ring into vagina; replace every 90 days |
Although vaginal estrogen has minimal systemic absorption; does not increase the risk of breast cancer, endometrial cancer, or venous thromboembolism9,10; and does not require concurrent use of progesterone for patients with an intact uterus, patients and physicians still may wish to avoid estrogen. This is often the case with patients who have a history of hormone-sensitive cancer, and it is good practice to consult the patient's oncologist before prescribing any hormonal medication for patients who have had breast cancer.
Alternatives to vaginal estrogen include intravaginal prasterone (Intrarosa), a synthetic form of dehydroepiandrosterone that is approved by the U.S. Food and Drug Administration (FDA) for the treatment of dyspareunia in postmenopausal patients. Prasterone also may be considered for treatment of other GSM symptoms, including moderate to severe vulvovaginal atrophy.11 Ospemifene (Osphena), an oral selective estrogen receptor modulator that is FDA-approved for the treatment of moderate to severe dyspareunia, is also effective for the treatment of vaginal dryness.12
Fractional carbon-dioxide laser therapy creates microabrasions that stimulate blood flow and thicken vaginal tissue. Small studies indicate that it is as effective as vaginal estrogen in treating symptoms of GSM.13–16 However, patients should be cautioned that long-term risks of this procedure are not known, and that they should seek treatment only from physicians experienced with this procedure.
Lichen Sclerosus
Lichen sclerosus is a chronic inflammatory dermatitis that typically occurs in the vulvar and anogenital regions. It is associated with autoimmune diseases and hypoestrogenic states such as prepuberty and postmenopause.17,18 The prevalence is estimated to be 1.7%, with an incidence of 14 to 22 cases per 100,000 person-years.19–21
PRESENTATION
Patients with lichen sclerosus often experience severe pruritus and dyspareunia. They occasionally report burning, dysuria, or anal discomfort.18
DIAGNOSIS
Lichen sclerosis is characterized by epithelial thinning and inflammation with white, thin plaques on the vulvar skin18 (Figure 222); these plaques commonly occur in a figure-eight configuration involving the perineum and perianal region. Loss of vulvar architecture can occur in advanced disease, in which the labia minora regress and the clitoris becomes scarred or buried beneath the clitoral hood (clitoral phimosis).
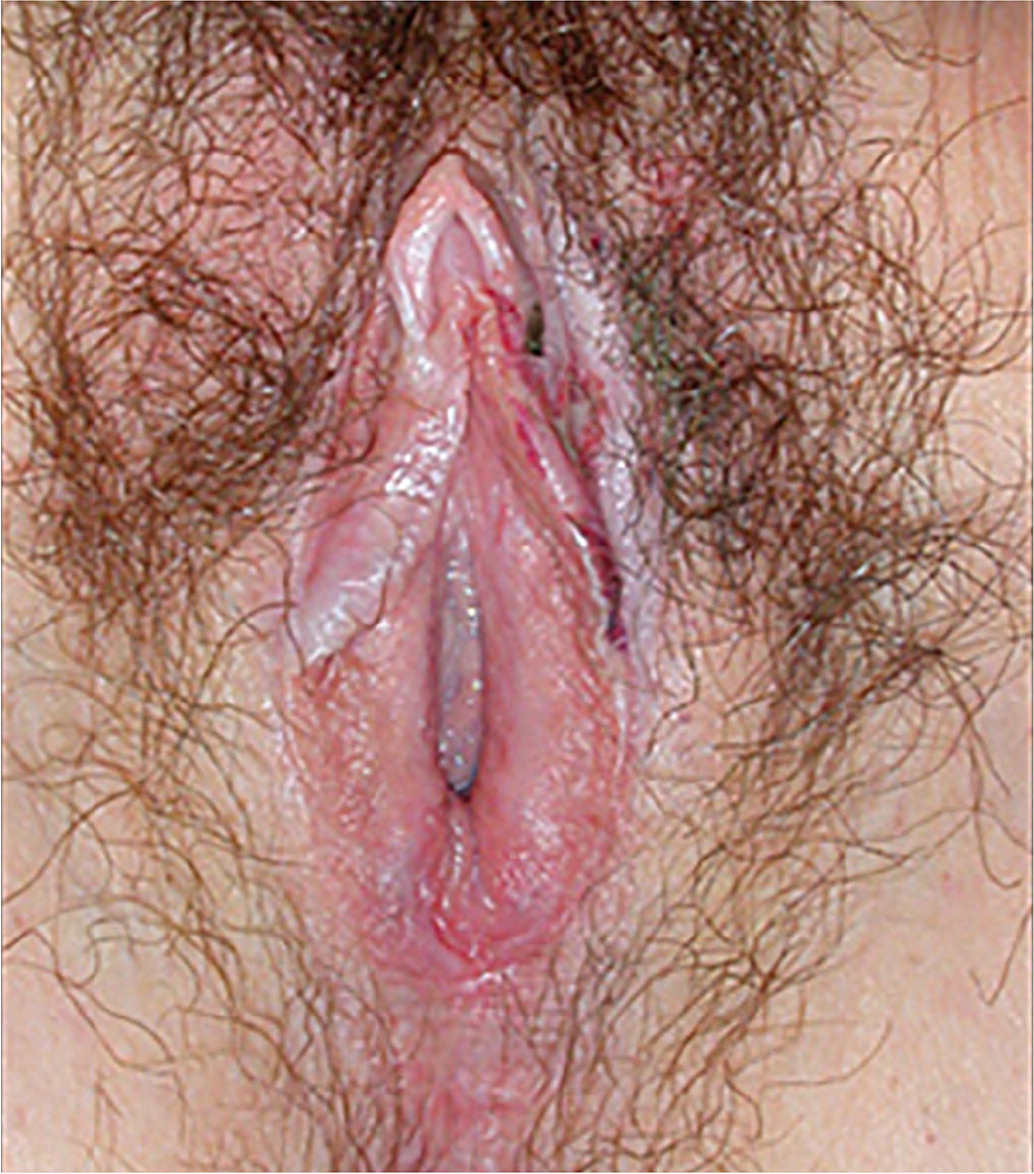
The workup for suspected lichen sclerosus involves ruling out other causes of symptoms, including infections. Because patients with lichen sclerosus are at risk of squamous cell carcinoma, a definitive diagnosis should be made with vulvar biopsy.20
TREATMENT
Patients with lichen sclerosus should be treated with a high-potency steroid ointment to alleviate symptoms, prevent architectural damage, and reverse histologic changes.23–26 Ointments are generally preferred over creams because of their barrier effects and reduced irritation. Clobetasol 0.05% ointment (Temovate) is most commonly used. It should be applied twice per day until active lesions have regressed (usually about one to two months). After regression, clobetasol can be tapered to one or two times per week for an additional two months, or the patient can be switched to a moderate-potency steroid such as betamethasone 0.1% ointment or other vulvar soothing cream.23
The goal of steroid therapy is to control symptoms and manifestations of disease while using the lowest possible dosage. However, multiple or long courses of topical steroids may be required for patients with lichen sclerosus. Although there are concerns that long-term topical steroid use may cause skin atrophy or irritation and lead to skin breakdown, evidence suggests that there are minimal adverse effects with prolonged or continued use.24 Follow-up visits should occur at least every six months to ensure disease stability. Biopsy should be repeated if worsening or changing lesions are noted, because there is a 4% to 5% risk of vulvar squamous cell carcinoma developing in lichen sclerosus lesions.20,25
If topical steroids are not effective, twice-daily application of a topical calcineurin inhibitor, such as pimecrolimus (Elidel) 1% cream, is an effective alternative.27 Intralesional injections with triamcinolone acetonide (no more than 40 mg across the entire vulva), oral or topical retinoids, oral cyclosporine (Sandimmune), oral methotrexate, oral hydroxyurea (Hydrea), phototherapy, fractional CO2 laser therapy, and mixed methylene blue injections are other options to consider.26,28–32
Lichen Planus
PRESENTATION
Lichen planus can affect any skin or mucosal surface. It often affects more than one part of the body, and many patients with oral manifestations also have genital involvement.36 Common vulvar symptoms include pain, burning, and scarring.
DIAGNOSIS
Physical examination may reveal well-demarcated, bright-red vestibular patches, a hyperkeratotic border, or a net-like plaque known as Wickham striae35 (Figure 322). Vulvar architecture may regress, and the vagina is typically affected when there is vulvar involvement.35 The workup involves ruling out other causes, but a definitive diagnosis should be made with biopsy.37
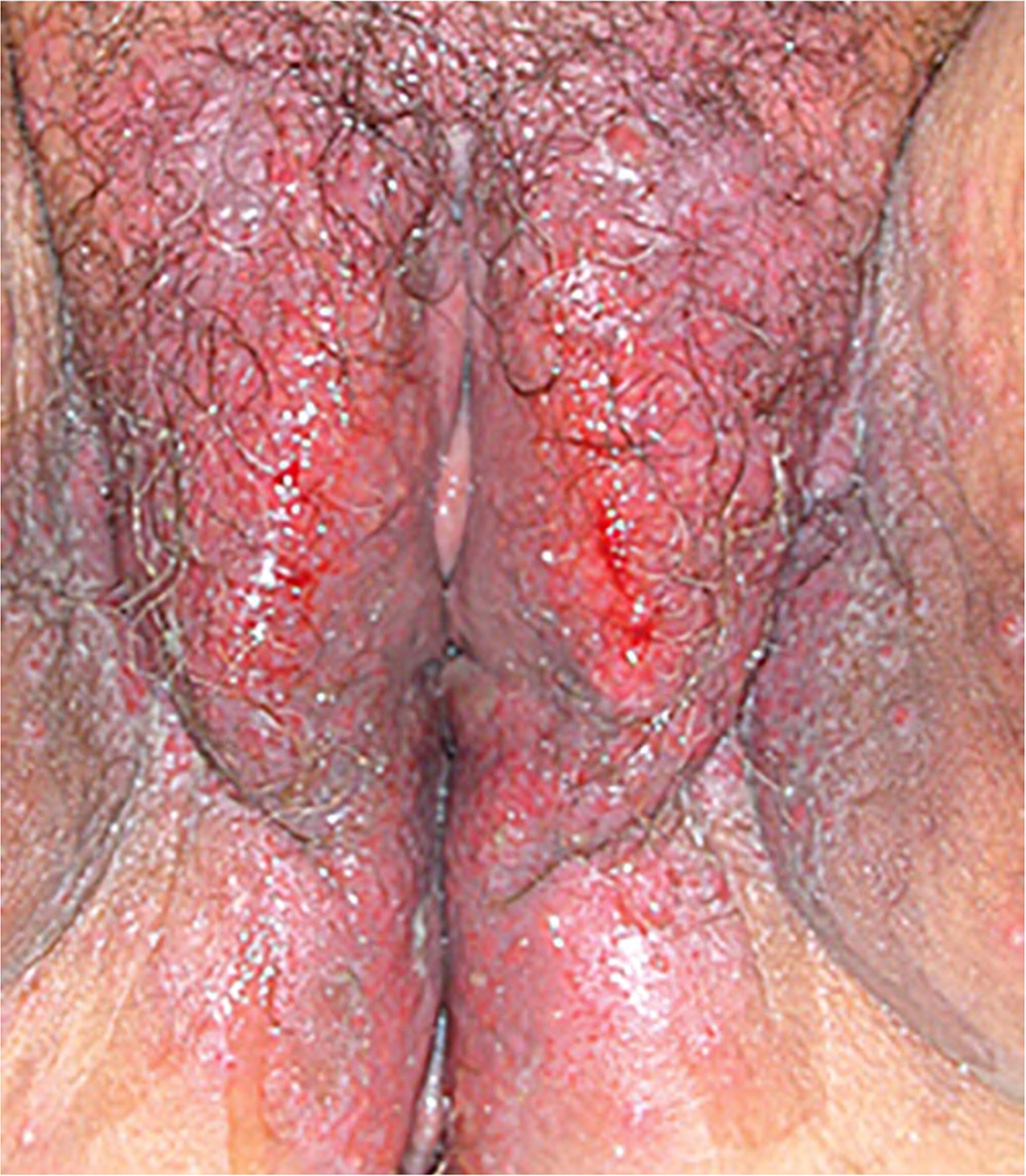
TREATMENT
First-line therapy for lichen planus is similar to that for lichen sclerosus, with twice-daily applications of high-dose steroid ointment (clobetasol 0.05%). Symptoms typically diminish with treatment but rarely resolve completely, often waxing and waning over time.35 The goal of treatment should be to improve symptoms and limit scarring, because eroded surfaces can adhere and lead to synechiae that gradually close the vaginal space. For this reason, intravaginal suppositories of hydrocortisone acetate, 25 mg, are recommended as a symptomatic treatment and to prevent scarring,38 and dilators coated in corticosteroid ointment can be considered for short-term use.
Topical calcineurin inhibitors can be considered for steroid-refractory cases, although frequent recurrence has been reported with this treatment.39,40 Other treatments such as systemic corticosteroids, immune modulators, antifungals, antimetabolites, methotrexate, and oral retinoids have been tried with varied, though often unsuccessful, results.2,41
Lichen Simplex Chronicus
Lichen simplex chronicus (LSC) is a secondary skin disorder that involves lichenification of the vulvar skin due to chronic pruritus and excoriation. LSC is a localized atopic dermatitis in about 65% to 75% of cases and is often associated with allergic conditions such as asthma, hay fever, and eczema.2 Its prevalence is estimated at approximately 0.5%, and it is more common in people older than 60 years and in those with anxiety disorders.42
PRESENTATION AND DIAGNOSIS
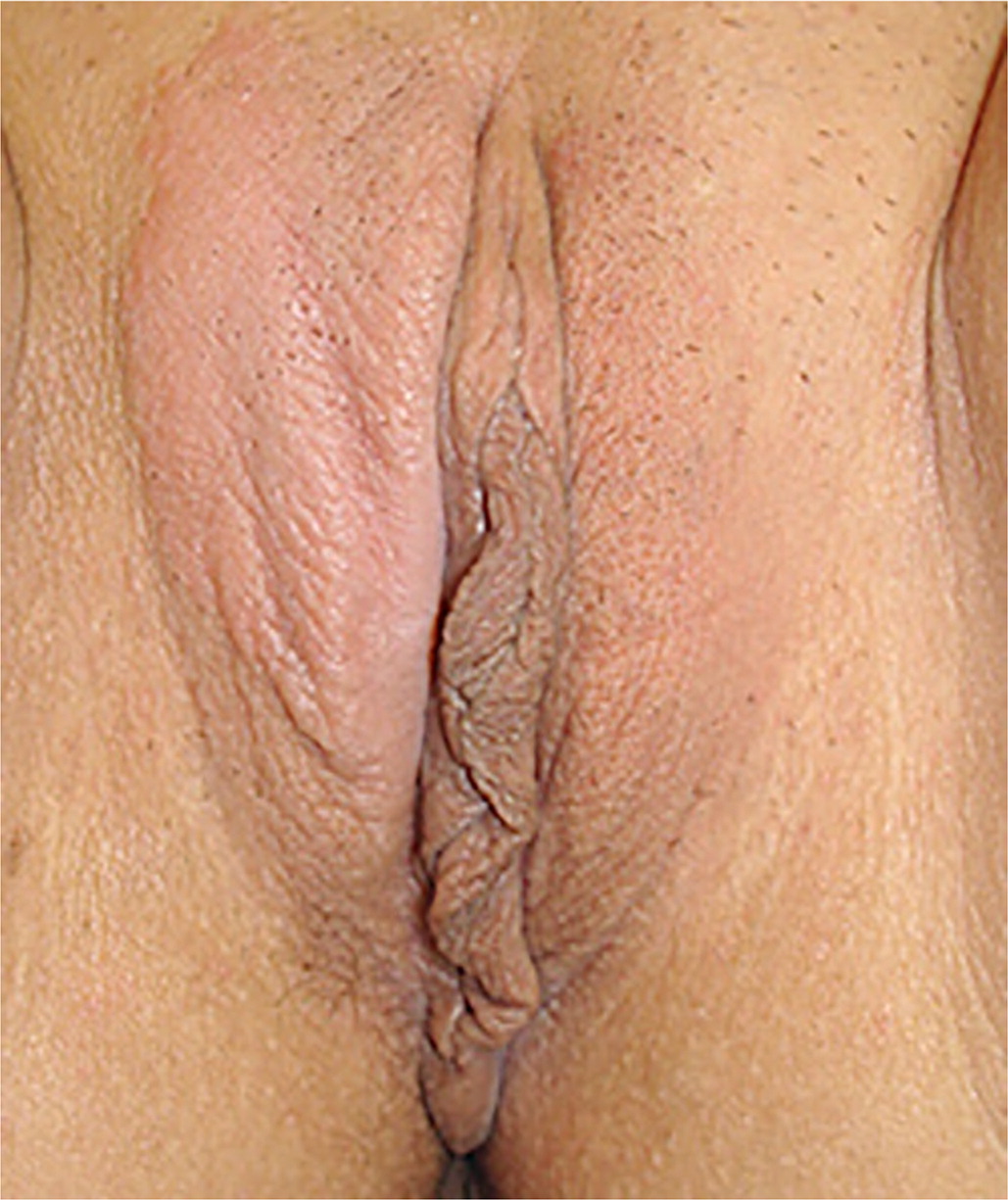
TREATMENT
The first step in the treatment of LSC is to consider whether an underlying infection (e.g., candidiasis) or disease (e.g., lichen sclerosus, lichen planus) is causing the intense itching; if so, that condition should be treated first. Avoidance of irritants such as harsh soaps, rough clothing, excessive washing, and daily wear of sanitary pads is also important.
If no underlying condition is identified, the key to therapy is breaking the itch-scratch cycle.2 The use of topical corticosteroids is thought to be the most effective treatment. Moderate-potency formulations (e.g., triamcinolone 0.1% ointment applied twice per day) can be used; a two- to four-week course of a high-potency steroid is another option.43,44 Treatment options for nocturnal itching include the anticholinergics hydroxyzine (starting at a dosage of 25 mg each night) and amitriptyline (25 to 50 mg nightly). These medications should generally be avoided in older adults because of their potential adverse cognitive effects. Systemic corticosteroids or calcineurin inhibitors can be considered for severe refractory cases.43
Vulvodynia
Vulvodynia is vulvar pain without an identifiable cause that persists for at least three months.45 Causes of vulvar pain that should be ruled out include infections (e.g., herpes simplex virus, candidiasis), trauma, neoplasia (e.g., Paget disease, vulvar intraepithelial neoplasia, squamous cell carcinoma), iatrogenic causes (e.g., radiation, chemotherapy, prior surgery), or any of the previously discussed conditions. Although the cause of pain in vulvodynia is not known, it is thought to begin after an inciting event that leads to inflammation and subsequent remodeling of nerve fibers that alters the pain response.45
Vulvodynia can occur alone or in association with another vulvar disorder. Potential comorbidities include interstitial cystitis, fibromyalgia, irritable bowel syndrome, pelvic myofascial pain, psychosocial conditions, and sexual dysfunction.46
PRESENTATION
Pain associated with vulvodynia varies and can be localized, generalized, or mixed; spontaneous or provoked; and intermittent, persistent, constant, immediate, or delayed. The condition is classified as primary (symptoms have always been present) or secondary (symptoms develop after a period of normal function). Vulvodynia is the most common cause of dyspareunia in premenopausal patients.47 Its prevalence is estimated at 12%, but only an estimated one-half of affected individuals seek treatment, and most are incorrectly diagnosed when they do.47
DIAGNOSIS
Diagnosis is based on clinical evaluation after other causes of pain have been ruled out. Physical examination should incorporate pressure point testing using a cotton swab in a circumferential fashion along the vulvar vestibule, which will elicit pain with even light touch in a patient with vulvodynia (Figure 5). Specific neuropathies (e.g., pudendal, ilioinguinal, genitofemoral) should be ruled out.
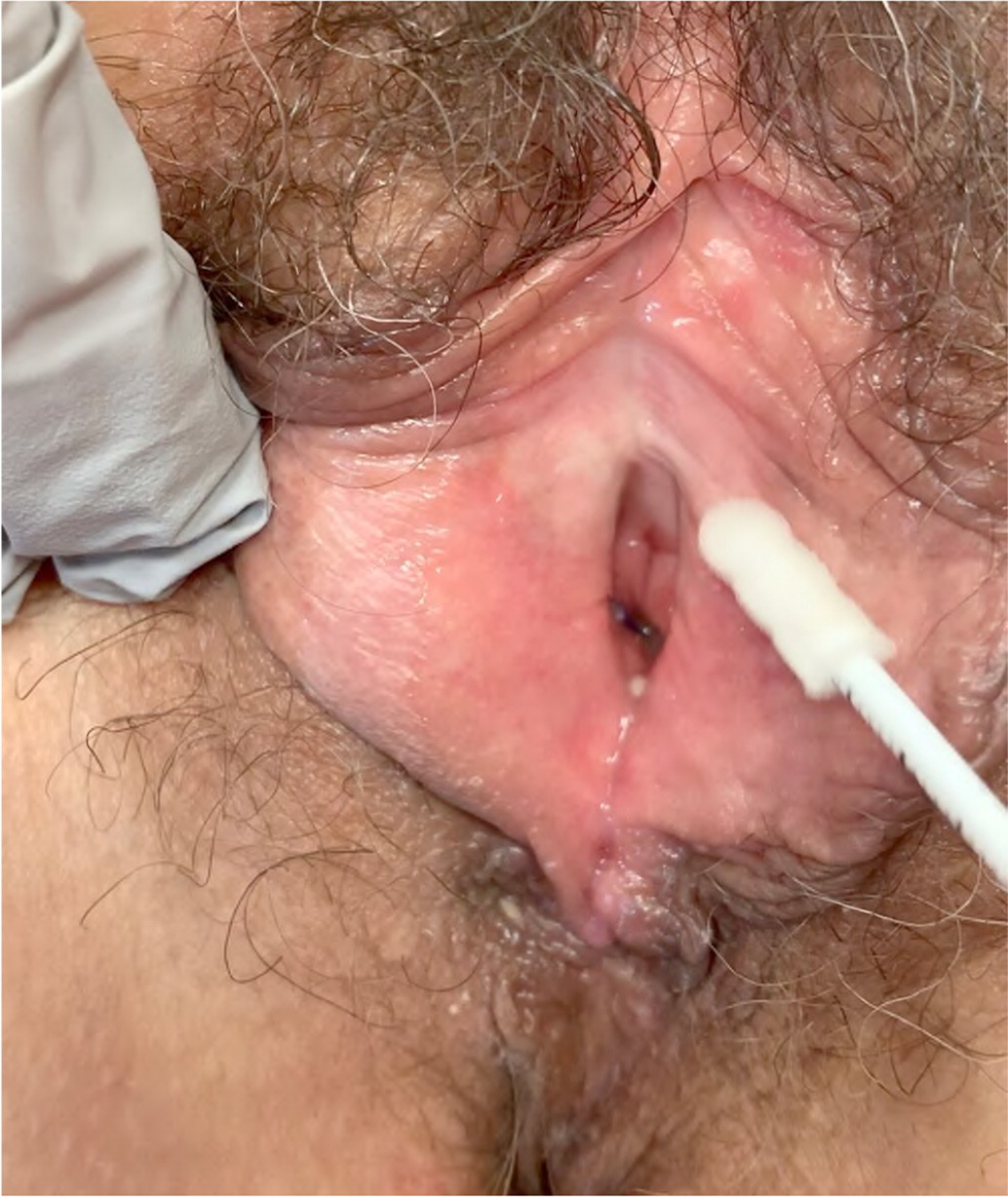
TREATMENT
Treatment of vulvodynia begins with simple changes in vulvar hygiene (e.g., avoiding tight clothing, use of mild soaps), stress reduction, pelvic floor physical therapy, and psychosocial interventions such as cognitive behavior therapy or mindfulness-based stress reduction.48 In addition to these physical and psychosocial treatments, a multimodal approach to therapy may include oral medications that alter nerve conduction (e.g., antidepressants, anticonvulsants) and the use of vaginal dilators.48 Topical medications (e.g., lidocaine ointment, estrogen and/or testosterone cream) are not recommended because they have not been proven superior to placebo.49 Vestibulectomy may be necessary in severe cases of localized vulvodynia; when it is performed, patients report improvement in symptoms.48,50
Vulvar Manifestations of Systemic Illness
Dermatologic and systemic conditions such as psoriasis, eczema, and Crohn disease can have vulvar manifestations. Although these conditions are less common causes of vulvar symptoms, they should remain in the differential diagnosis unless another diagnosis is confirmed. Biopsy can rule in or rule out these other conditions and is recommended if the diagnosis is uncertain.43 Table 2 provides a recommended approach to performing vulvar biopsies.
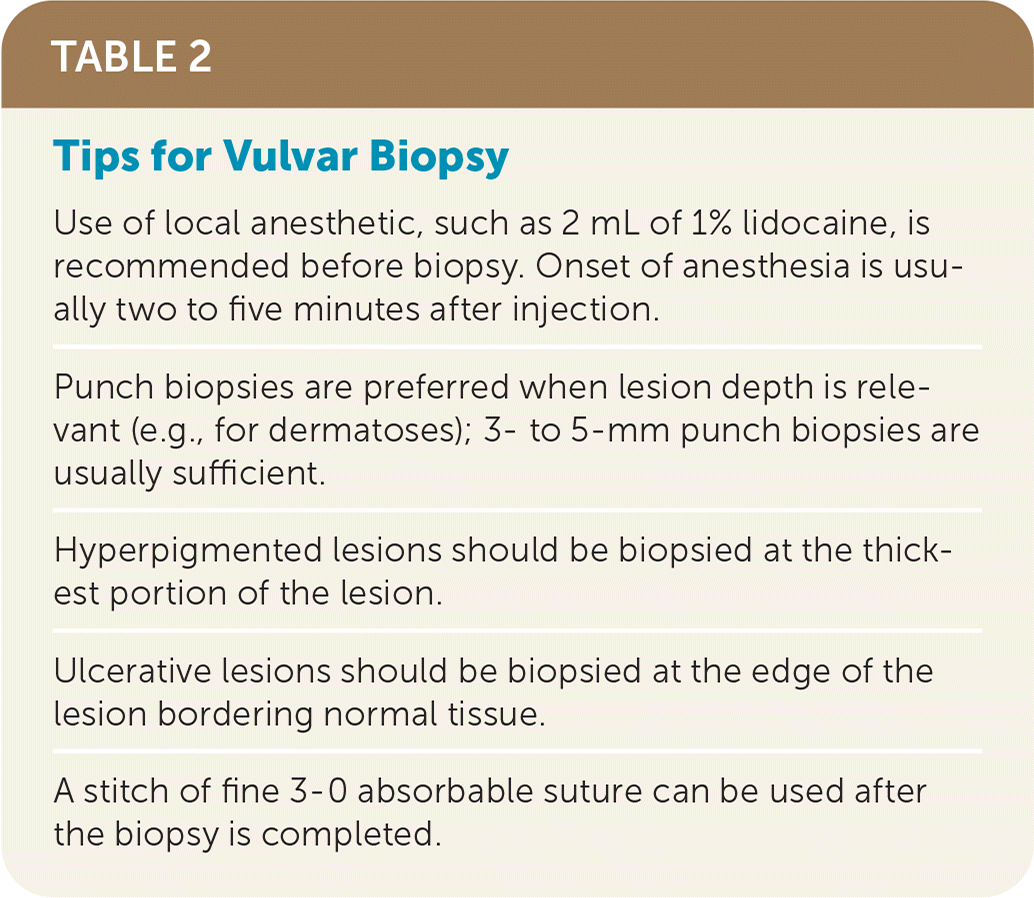
| Use of local anesthetic, such as 2 mL of 1% lidocaine, is recommended before biopsy. Onset of anesthesia is usually two to five minutes after injection. |
| Punch biopsies are preferred when lesion depth is relevant (e.g., for dermatoses); 3- to 5-mm punch biopsies are usually sufficient. |
| Hyperpigmented lesions should be biopsied at the thickest portion of the lesion. |
| Ulcerative lesions should be biopsied at the edge of the lesion bordering normal tissue. |
| A stitch of fine 3-0 absorbable suture can be used after the biopsy is completed. |
This article updates a previous article on this topic by O'Connell, et al.22
Data Sources: A PubMed search was completed in Clinical Queries using the key terms vulvar disorders, vulvar dermatoses, benign vulvar conditions, vulvar pathology, lichen sclerosus, lichen planus, lichen simplex chronicus, genitourinary syndrome of menopause, vulvodynia, vulvar pruritus, and vulvar pain. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. Also searched were the Cochrane database and the Agency for Healthcare Research and Quality Effective Healthcare Reports. Search dates: September 2, 2019; October 18, 2019; and May 23, 2020.
The authors thank Emory Buck, MD, for research assistance for this article.
