
Am Fam Physician. 2020;102(9):613-621
Author disclosure: No relevant financial affiliations.
Drugs are being prescribed with more frequency and in higher quantities. A serious adverse drug event from prescribed medications constitutes 2.4% to 16.2% of all hospital admissions. Many of the adverse drug events present intraorally or periorally in isolation or as a clinical symptom of a systemic effect. Clinical recognition and treatment of adverse drug events are important to increase patient adherence, manage drug therapy, or detect early signs of potentially serious outcomes. Oral manifestations of commonly prescribed medications include gingival enlargement, oral hyperpigmentation, oral hypersensitivity reaction, medication-related osteonecrosis, xerostomia, and other oral or perioral conditions. To prevent dose-dependent adverse drug reactions, physicians should prescribe medications judiciously using the lowest effective dose with minimal duration. Alternatively, for oral hypersensitivity reactions that are not dose dependent, quick recognition of clinical symptoms associated with time-dependent drug onset can allow for immediate discontinuation of the medication without discontinuation of other medications. Physicians can manage oral adverse drug events in the office through oral hygiene instructions for gingival enlargement, medication discontinuation for oral pigmentation, and prescription of higher fluoride toothpastes for xerostomia.
Drugs are being prescribed with more frequency and in higher quantities per patient, with an estimated prevalence of 59% of the U.S. population taking at least one prescription medication and 15% taking five or more prescription drugs.1 A serious adverse drug event from prescribed medications constitutes 2.4% to 16.2% of all hospital admissions with an estimated treatment cost of $1.6 to $5.6 billion.2 Increased usage of medications leads to increased adverse drug reactions, particularly in vulnerable elderly populations.3 All of the 10 most commonly filled prescription medications4 have adverse effects associated with the orofacial complex, including hypersensitivity type reactions, xerostomia, paresthesia, and dysgeusia5 (Table 13–5). Other adverse reactions, such as vomiting, can cause secondary oral symptoms of tooth erosion, oral mucosal erythema or edema, and subsequent increase in dental caries. Adverse effects can present as severe systemic disease, for example Stevens-Johnson syndrome. Close attention is warranted even in seemingly mild adverse effects, such as xerostomia, because these might decrease patient compliance with prescribed drug therapies or have negative effects on quality of life.6 A high suspicion for medication adverse effects as a source of oral complaints can thus lead to appropriate interventions, such as stopping the causative medication, finding appropriate substitutes, and identifying serious reactions in a timely manner. The main categories of oral or perioral manifestations attributable to prescribed medications are gingival enlargement, oral hyperpigmentation, oral hypersensitivity reaction, osteonecrosis, xerostomia, and other oral or perioral conditions such as angioedema and chemical burns (Table 2).
| Clinical recommendation | Evidence rating | Comments |
|---|---|---|
| Minimizing intraoral plaque can reduce the incidence of medication-induced gingival enlargement.7,24,25,30,31 | B | Systematic reviews of lower quality clinical trials and expert opinion |
| If oral hyperpigmentation does not resolve with discontinuation of the offending medication, surgical laser therapy is a treatment option.38 | B | Based on a systematic review |
| Before starting antiresorptive therapy, patients should be counseled on maintaining good oral hygiene, routine dental visits, and tobacco cessation.52 | C | Based on systematic review of clinical practice guidelines |
| Xerostomia is typically managed with conservative therapy and judicious use of medications; oral pilocarpine is effective in patients with persistent symptoms.61–63 | B | Based on a systematic review and two cohort studies |
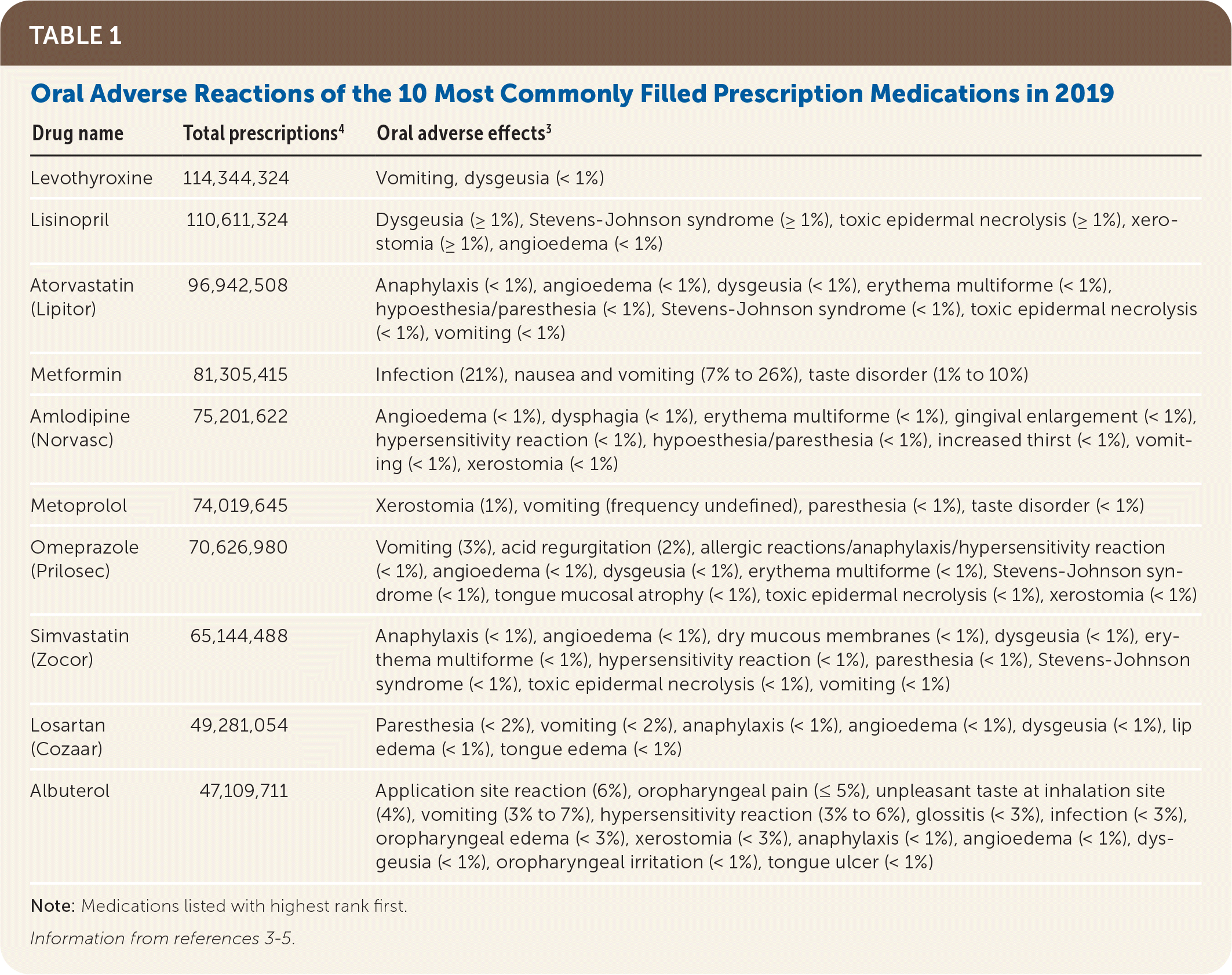
| Drug name | Total prescriptions4 | Oral adverse effects3 |
|---|---|---|
| Levothyroxine | 114,344,324 | Vomiting, dysgeusia (< 1%) |
| Lisinopril | 110,611,324 | Dysgeusia (≥ 1%), Stevens-Johnson syndrome (≥ 1%), toxic epidermal necrolysis (≥ 1%), xerostomia (≥ 1%), angioedema (< 1%) |
| Atorvastatin (Lipitor) | 96,942,508 | Anaphylaxis (< 1%), angioedema (< 1%), dysgeusia (< 1%), erythema multiforme (< 1%), hypoesthesia/paresthesia (< 1%), Stevens-Johnson syndrome (< 1%), toxic epidermal necrolysis (< 1%), vomiting (< 1%) |
| Metformin | 81,305,415 | Infection (21%), nausea and vomiting (7% to 26%), taste disorder (1% to 10%) |
| Amlodipine (Norvasc) | 75,201,622 | Angioedema (< 1%), dysphagia (< 1%), erythema multiforme (< 1%), gingival enlargement (< 1%), hypersensitivity reaction (< 1%), hypoesthesia/paresthesia (< 1%), increased thirst (< 1%), vomiting (< 1%), xerostomia (< 1%) |
| Metoprolol | 74,019,645 | Xerostomia (1%), vomiting (frequency undefined), paresthesia (< 1%), taste disorder (< 1%) |
| Omeprazole (Prilosec) | 70,626,980 | Vomiting (3%), acid regurgitation (2%), allergic reactions/anaphylaxis/hypersensitivity reaction (< 1%), angioedema (< 1%), dysgeusia (< 1%), erythema multiforme (< 1%), Stevens-Johnson syndrome (< 1%), tongue mucosal atrophy (< 1%), toxic epidermal necrolysis (< 1%), xerostomia (< 1%) |
| Simvastatin (Zocor) | 65,144,488 | Anaphylaxis (< 1%), angioedema (< 1%), dry mucous membranes (< 1%), dysgeusia (< 1%), erythema multiforme (< 1%), hypersensitivity reaction (< 1%), paresthesia (< 1%), Stevens-Johnson syndrome (< 1%), toxic epidermal necrolysis (< 1%), vomiting (< 1%) |
| Losartan (Cozaar) | 49,281,054 | Paresthesia (< 2%), vomiting (< 2%), anaphylaxis (< 1%), angioedema (< 1%), dysgeusia (< 1%), lip edema (< 1%), tongue edema (< 1%) |
| Albuterol | 47,109,711 | Application site reaction (6%), oropharyngeal pain (≤ 5%), unpleasant taste at inhalation site (4%), vomiting (3% to 7%), hypersensitivity reaction (3% to 6%), glossitis (< 3%), infection (< 3%), oropharyngeal edema (< 3%), xerostomia (< 3%), anaphylaxis (< 1%), angioedema (< 1%), dysgeusia (< 1%), oropharyngeal irritation (< 1%), tongue ulcer (< 1%) |
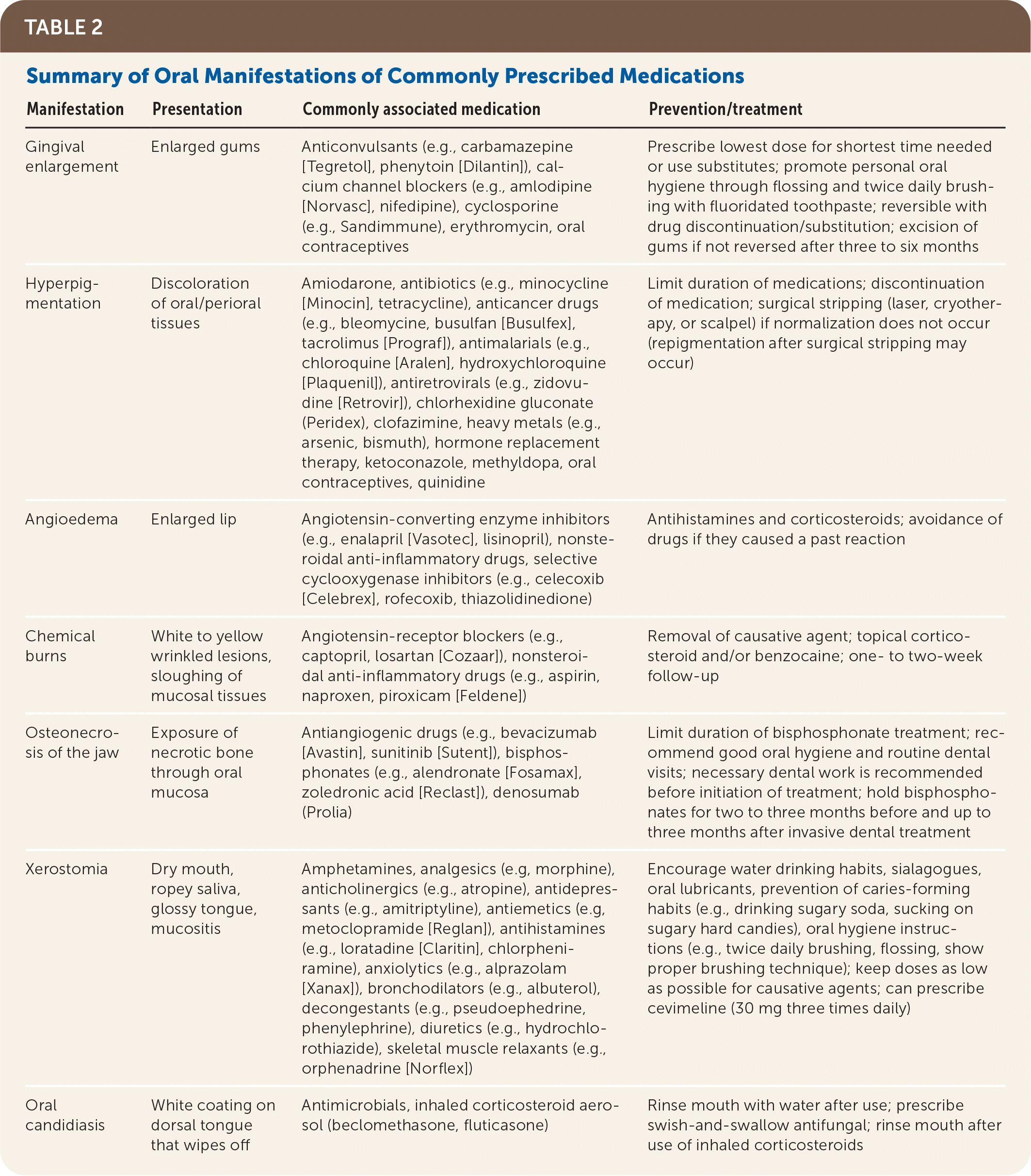
| Manifestation | Presentation | Commonly associated medication | Prevention/treatment |
|---|---|---|---|
| Gingival enlargement | Enlarged gums | Anticonvulsants (e.g., carbamazepine [Tegretol], phenytoin [Dilantin]), calcium channel blockers (e.g., amlodipine [Norvasc], nifedipine), cyclosporine (e.g., Sandimmune), erythromycin, oral contraceptives | Prescribe lowest dose for shortest time needed or use substitutes; promote personal oral hygiene through flossing and twice daily brushing with fluoridated toothpaste; reversible with drug discontinuation/substitution; excision of gums if not reversed after three to six months |
| Hyperpigmentation | Discoloration of oral/perioral tissues | Amiodarone, antibiotics (e.g., minocycline [Minocin], tetracycline), anticancer drugs (e.g., bleomycine, busulfan [Busulfex], tacrolimus [Prograf]), antimalarials (e.g., chloroquine [Aralen], hydroxychloroquine [Plaquenil]), antiretrovirals (e.g., zidovudine [Retrovir]), chlorhexidine gluconate (Peridex), clofazimine, heavy metals (e.g., arsenic, bismuth), hormone replacement therapy, ketoconazole, methyldopa, oral contraceptives, quinidine | Limit duration of medications; discontinuation of medication; surgical stripping (laser, cryotherapy, or scalpel) if normalization does not occur (repigmentation after surgical stripping may occur) |
| Angioedema | Enlarged lip | Angiotensin-converting enzyme inhibitors (e.g., enalapril [Vasotec], lisinopril), nonsteroidal anti-inflammatory drugs, selective cyclooxygenase inhibitors (e.g., celecoxib [Celebrex], rofecoxib, thiazolidinedione) | Antihistamines and corticosteroids; avoidance of drugs if they caused a past reaction |
| Chemical burns | White to yellow wrinkled lesions, sloughing of mucosal tissues | Angiotensin-receptor blockers (e.g., captopril, losartan [Cozaar]), nonsteroidal anti-inflammatory drugs (e.g., aspirin, naproxen, piroxicam [Feldene]) | Removal of causative agent; topical corticosteroid and/or benzocaine; one- to two-week follow-up |
| Osteonecrosis of the jaw | Exposure of necrotic bone through oral mucosa | Antiangiogenic drugs (e.g., bevacizumab [Avastin], sunitinib [Sutent]), bisphosphonates (e.g., alendronate [Fosamax], zoledronic acid [Reclast]), denosumab (Prolia) | Limit duration of bisphosphonate treatment; recommend good oral hygiene and routine dental visits; necessary dental work is recommended before initiation of treatment; hold bisphosphonates for two to three months before and up to three months after invasive dental treatment |
| Xerostomia | Dry mouth, ropey saliva, glossy tongue, mucositis | Amphetamines, analgesics (e.g, morphine), anticholinergics (e.g., atropine), antidepressants (e.g., amitriptyline), antiemetics (e.g, metoclopramide [Reglan]), antihistamines (e.g., loratadine [Claritin], chlorpheniramine), anxiolytics (e.g., alprazolam [Xanax]), bronchodilators (e.g., albuterol), decongestants (e.g., pseudoephedrine, phenylephrine), diuretics (e.g., hydrochlorothiazide), skeletal muscle relaxants (e.g., orphenadrine [Norflex]) | Encourage water drinking habits, sialagogues, oral lubricants, prevention of caries-forming habits (e.g., drinking sugary soda, sucking on sugary hard candies), oral hygiene instructions (e.g., twice daily brushing, flossing, show proper brushing technique); keep doses as low as possible for causative agents; can prescribe cevimeline (30 mg three times daily) |
| Oral candidiasis | White coating on dorsal tongue that wipes off | Antimicrobials, inhaled corticosteroid aerosol (beclomethasone, fluticasone) | Rinse mouth with water after use; prescribe swish-and-swallow antifungal; rinse mouth after use of inhaled corticosteroids |
Gingival Enlargement
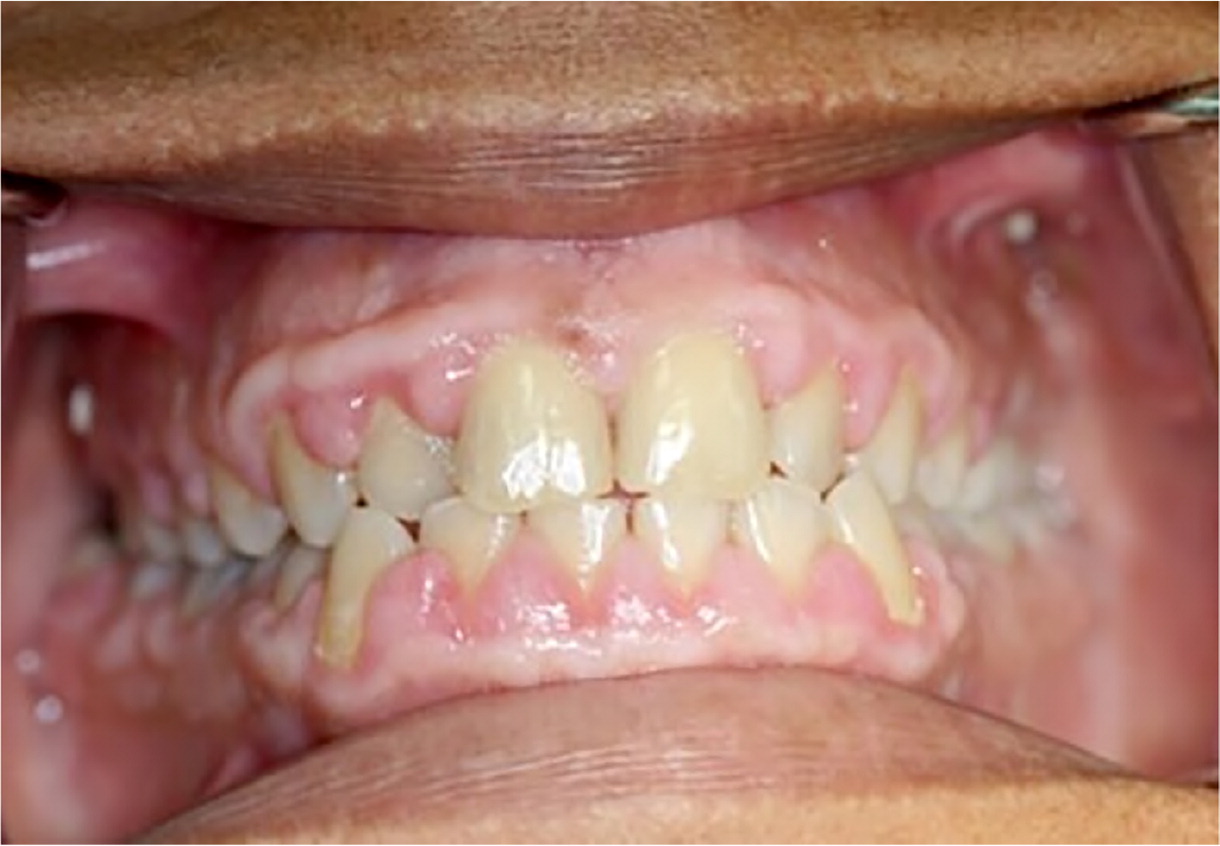
Gingival enlargement (or gingival hyperplasia/hypertrophy or overgrowth) is the enlargement of gum tissue in the mouth (Figure 1). Classic drugs associated with medication-induced gingival enlargement include calcium channel blockers, anticonvulsants, and cyclosporine (Sandimmune). Erythromycin and oral contraceptives have also been implicated in gingival enlargement. The increased overgrowth of gingival tissue is related to the disruption of the degradation of collagen, which leads to a larger amount of extracellular collagen tissue within the gums. Amlodipine (Norvasc), diltiazem, nifedipine, phenytoin (Dilantin), and verapamil are most commonly implicated in gingival enlargement7 (Table 37–23). It is well documented that the amount of plaque in the mouth is an independent risk factor in the prevalence of medication-induced gingival enlargement.7,24,25 Patients with gingival enlargement have an increased height of gingival tissue (measured from the tooth-gum connection to the top of the gums), creating a challenging environment for patients and professionals to clean plaque from the mouth. Therefore, meticulous plaque control is important to limit the extent and recurrence of the gingival enlargement. Other risk factors include being male (three times more likely), younger age (younger than 40 years),26 and taking higher daily doses of medication24 when there may be a possible genetic predisposition to gingival enlargement.27
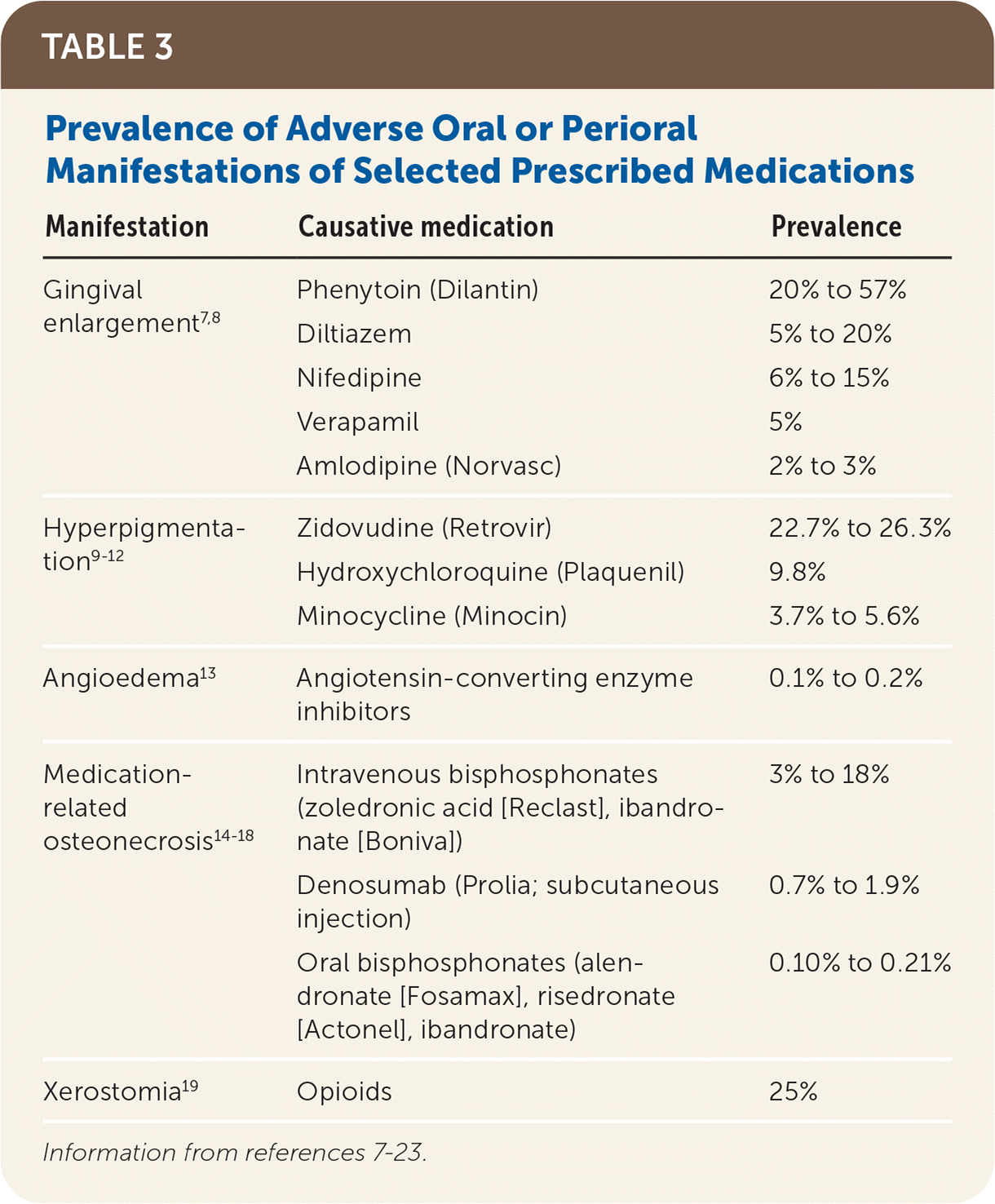
| Manifestation | Causative medication | Prevalence |
|---|---|---|
| Gingival enlargement7,8 | Phenytoin (Dilantin) | 20% to 57% |
| Diltiazem | 5% to 20% | |
| Nifedipine | 6% to 15% | |
| Verapamil | 5% | |
| Amlodipine (Norvasc) | 2% to 3% | |
| Hyperpigmentation9–12 | Zidovudine (Retrovir) | 22.7% to 26.3% |
| Hydroxychloroquine (Plaquenil) | 9.8% | |
| Minocycline (Minocin) | 3.7% to 5.6% | |
| Angioedema13 | Angiotensin-converting enzyme inhibitors | 0.1% to 0.2% |
| Medication-related osteonecrosis14–18 | Intravenous bisphosphonates (zoledronic acid [Reclast], ibandronate [Boniva]) | 3% to 18% |
| Denosumab (Prolia; subcutaneous injection) | 0.7% to 1.9% | |
| Oral bisphosphonates (alendronate [Fosamax], risedronate [Actonel], ibandronate) | 0.10% to 0.21% | |
| Xerostomia19 | Opioids | 25% |
Gingival enlargement can present as an aesthetic concern to patients resulting in poor patient compliance with the medication.28 Tissue enlargement starts one to three months after initiation of medication,29 and discontinuation of medication with substitution can decrease effects of medication-induced gingival enlargement. Reduction of gingival size typically occurs within six months to one year after discontinuation of the causative medication. The first, most ideal step to prevent gingival enlargement is plaque control by the patient.7,24,25,30,31 Treatment options include reducing dose and/or duration of the causative medication, reviewing oral hygiene instructions to the patient, increasing frequency of professional dental cleanings with adjunctive use of chlorhexidine gluconate (Peridex), using topical folic acid, open-flap debridement (reflecting gingival tissue to clean teeth), or removing excess tissue with laser, electrosurgery, or scalpel.25,32,33 Chlorhexidine gluconate is typically prescribed under the supervision of a dentist because of the potential adverse effect of oral hyperpigmentation with prolonged use.
Oral Hyperpigmentation
Acquired oral mucosa hyperpigmentation is the discoloration of oral tissues and tongue that is not secondary to a congenitally present physiologic discoloration. Multiple medications can cause acquired hyperpigmentation of the mucosa in the oral cavity, leading to a cosmetic concern. Drugs used to prevent and treat malaria (e.g., chloroquine [Aralen], hydroxychloroquine [Plaquenil]) can cause oral mucosa hyperpigmentation due to direct stimulation of melanocytes.11,34 In addition, zidovudine (Retrovir), oral contraceptives, and hormone replacement therapy indirectly affect an increase in alpha melanocyte–stimulating hormone with subsequent clinical presentation of oral or perioral pigmentation.35 Zidovudine causes mucosal hyperpigmentation in 22.7% to 26.3% of HIV cases9,10 (Figure 2). Physicians should never assume that hyperpigmentation is a medication adverse effect; oral melanoma— although rare—can manifest in a similar fashion but is more likely to be focal in an early stage.36
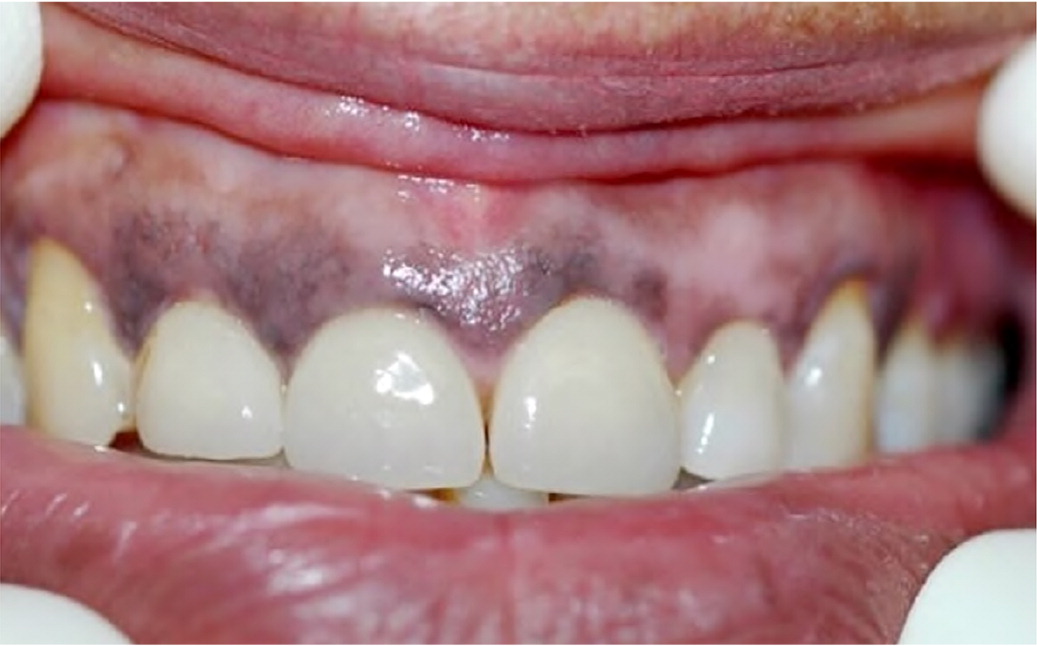
In addition to pigmentation within oral mucosa, medications can cause internal and external discoloration of the hard tissues (i.e., bone, teeth). A decreased usage of tetracycline class antibiotics in children younger than eight years has occurred because of current widespread knowledge that these antibiotics discolor developing teeth; therefore, internal staining of the bone and teeth is less commonly seen. Internal pigmentation of the bone, as seen with higher doses of minocycline (Minocin), can be detected under the thin mucosa of the mouth, which can give the appearance of discolored gingival tissue.37 Factors such as duration of medication use, location of hard tissue intrinsic staining, bone structure, and thick or thin mucosal biotype result in a varied clinical presentation. External staining of the teeth, as opposed to internal staining, can be removed through professional dental cleaning, involving abrasives, and can be caused by medications containing iron, iodine, sulfides, silver nitrate, manganese, copper, nickel, cadmium, essential oils, chlorhexidine (gluconate), amoxicillin/clavulanate (Augmentin), and doxycycline.13,23 Discontinuation of the offending medication can cause the oral pigmentation to disappear; however, in some cases, if normalization does not occur surgical interventions can be performed (e.g., laser, cryosurgery, scalpel).38–40
Oral Hypersensitivity Reaction
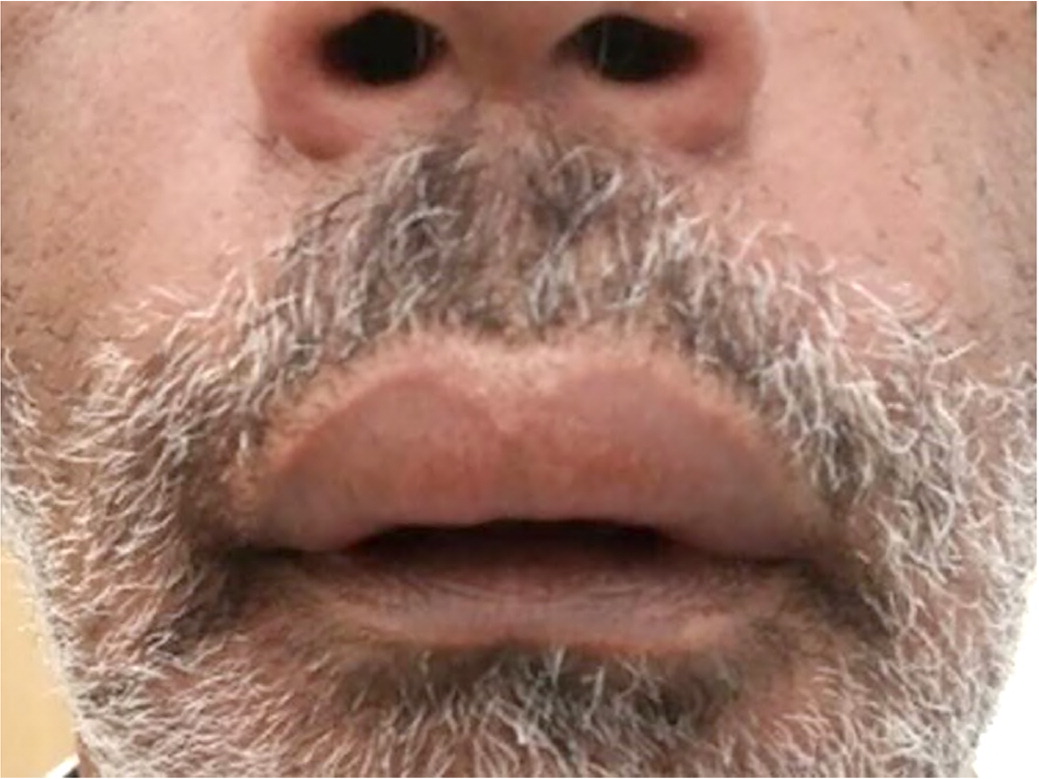
Allergic reactions presenting intra- or periorally because of a prescribed medication are not uncommon and, in most cases, are mild, with a wide host of clinical presentations. Allergic reactions of the oral cavity can include allergic contact stomatitis, angioedema, fixed-drug eruption, and erythema multiforme/Stevens-Johnson syndrome.41
Allergic contact stomatitis is a delayed-type hypersensitivity reaction mediated by T-cell immune reaction. Prescribed medications that might cause this reaction are chlorhexidine (gluconate), topical glucocorticoids, or inhaled budesonide (Rhinocort).42 Angioedema is typically an allergic immunoglobulin E (IgE)–dependent hypersensitivity in which localized swelling occurs because of increased vascular permeability41 (Figure 3). In the case of angiotensin-converting enzyme inhibitors, angioedema occurs because of impaired bradykinin breakdown.43 Fixed-drug eruptions are a T-cell mediated allergic inflammatory response to a systemic medication that recurs in the same mucosal or cutaneous location. Labial mucosa is the most frequent location of fixed-drug eruptions in the mouth.41 Stevens-Johnson syndrome, a severe immune-mediated condition, has an annual incidence of one to seven patients per million cases44 (Figure 4). The rapidly developing cutaneous and mucosal epithelial apoptosis results in life-threatening disorders with mortality rates of 9% for Stevens-Johnson syndrome and 45% for toxic epidermal necrolysis which is the most severe form of erythema multiforme.45 More than 200 medications have been associated with risks for Stevens-Johnson syndrome or toxic epidermal necrolysis,13 with the highest risk medications being antiepileptics (e.g., lamotrigine [Lamictal], carbamazepine [Tegretol], phenytoin, phenobarbital), sulfonamides, nevirapine (Viramune), allopurinol, and oxicam class nonsteroidal anti-inflammatory drugs.46 Oral and cutaneous reactions in Stevens-Johnson syndrome and toxic epidermal necrolysis most commonly occur four to 28 days after onset of the causative medication, depending on the medication's half-life.46 The causative medication should be discontinued immediately without needing to discontinue other medications.47
Medication-Related Osteonecrosis
Medication-related osteonecrosis occurs most commonly in the mandible, yet in rare cases can present extraorally or on the maxilla. The alveolar process (the portion of the jawbone that houses the teeth) is constantly remodeling through resorption (osteoclast activity) and apposition (osteoblast activity). The risk of osteonecrosis is increased in patients receiving drugs that cause osteoclast apoptosis, inhibit osteoclastic activity, or reduce blood flow that supports bone remodeling processes. The mandibular alveolar process is at increased risk for medication-induced osteonecrosis because this area has a high amount of remodeling at baseline and is exposed to procedures (i.e., tooth extraction) that require bone remodeling. Approximately 45% to 61% of medication-induced osteonecrosis cases report tooth removal as the precipitating event; however, 17% to 70% report a spontaneous precipitating event.48 A spontaneous cause of medication-induced osteonecrosis occurs without a known precipitating cause, but it is possible that many of these incidents could result from patient-induced minor localized trauma to the gingiva.48 Intravenous bisphosphonates, typically for antineoplastic treatment, have the highest incidence of osteonecrosis (3% to 18%),14,15 whereas the use of oral bisphosphonates for the prevention and treatment of osteoporosis results in an incidence of osteonecrosis of 0.10% to 0.21%.18 Clinical signs of osteonecrosis include radiolucency on radiographic imaging, exposed necrotic bone, socket remains after dental extraction, extra- or intraoral fistula, pain, suppuration, bone or gingival swelling, and sloughing of mucosa49 (Figure 5).
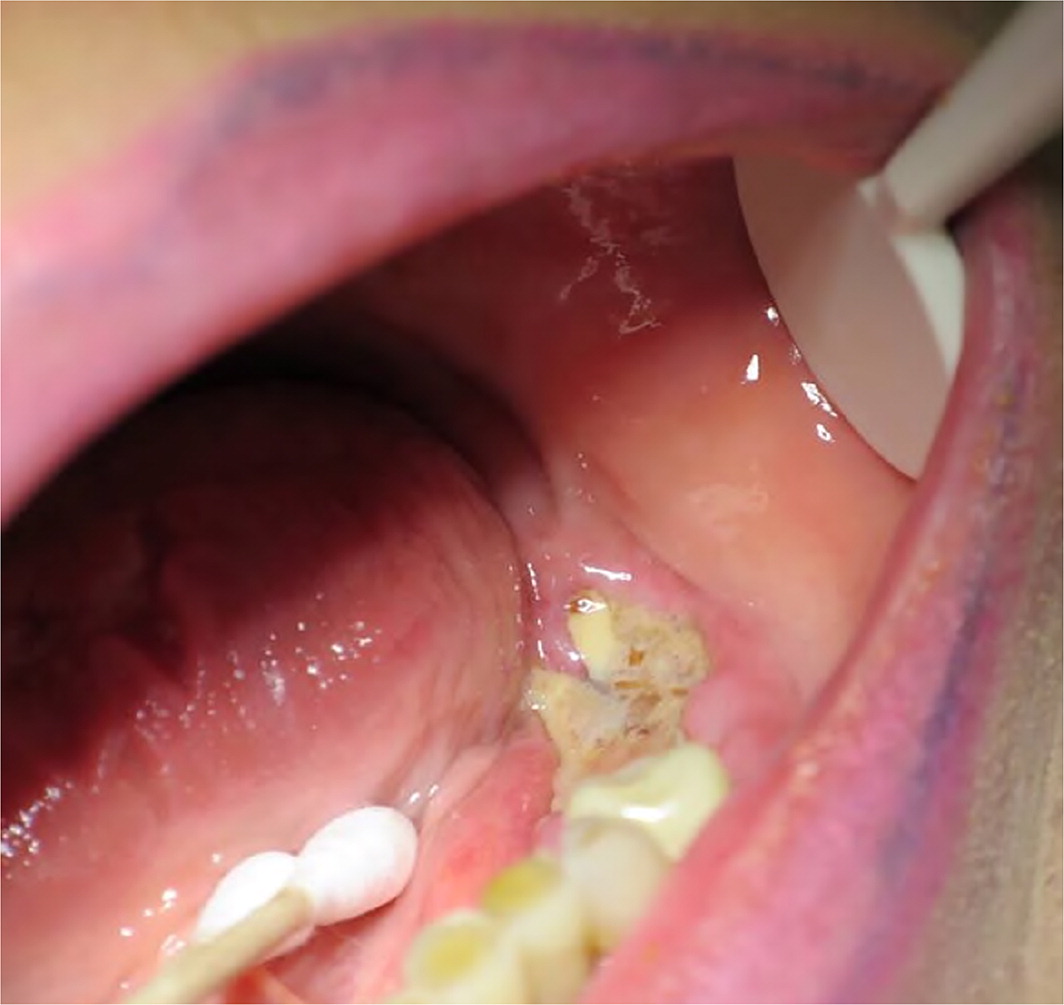
Treatment of medication-induced osteonecrosis remains a challenge for dental practitioners. Current literature has not supported a benefit associated with drug holiday or cessation of bisphosphonates before dental treatment and subsequent to dental surgery (although a two-month drug holiday before and up to a three-month drug holiday after an invasive dental procedure for patients receiving bisphosphonates in duration of more than four years is the current standard of practice50) or hyperbaric oxygen to prevent nonspontaneous osteonecrosis.51 Before starting antiresorptive therapy, patients should be counseled on maintaining good oral hygiene, routine dental visits, and tobacco cessation.52 Clearance for dental surgeries is recommended if a patient took oral bisphosphonates for four years or more. Dental surgery should be avoided if the patient is receiving intravenous bisphosphonates or antiangiogenic medications used for the treatment of cancer.52
Xerostomia
Xerostomia is the perceived sensation of a dry mouth. The parotid, submandibular, and sublingual salivary glands account for most of the salivary production. Patients stating that they have dry mouth does not necessitate that dysfunction of the salivary glands has occurred. Alternatively, salivary dysfunction could be attributable to radiation therapy, Sjögren syndrome, or another nonmedication-induced factor. In certain situations, salivary dysfunction can be measured in the office using salivary flow rates. Hyposalivation is considered less than 0.5 to 0.7 mL per minute of stimulated salivation and 0.1 mL per minute of unstimulated salivation.53 Medications affect the unstimulated flow rate to a greater degree than the stimulated flow rate.54 Clinical signs of xerostomia include, but are not limited to, tongue and cheeks sticking to oral mucosa, frothy or ropey saliva, lack of saliva pooling, atrophy of the filiform papilla or smooth appearance of the dorsal tongue, fissured tongue, oral candidiasis (thrush), and presence of dental caries (Figure 6 and Figure 7). Saliva is important in the function of buffering pH in the mouth; diluting hot, cold, and spicy foods; preventing tooth decay; preventing oral candidiasis and the proliferation of other oral microbes; preventing halitosis; promoting wound healing; preventing dysgeusia; speaking; chewing; and swallowing. Given the variety of functions of saliva, it is not surprising that drug-induced xerostomia is frequently associated with reports of other mucosal alterations such as mouth soreness, burning mouth, halitosis, and taste alterations.55
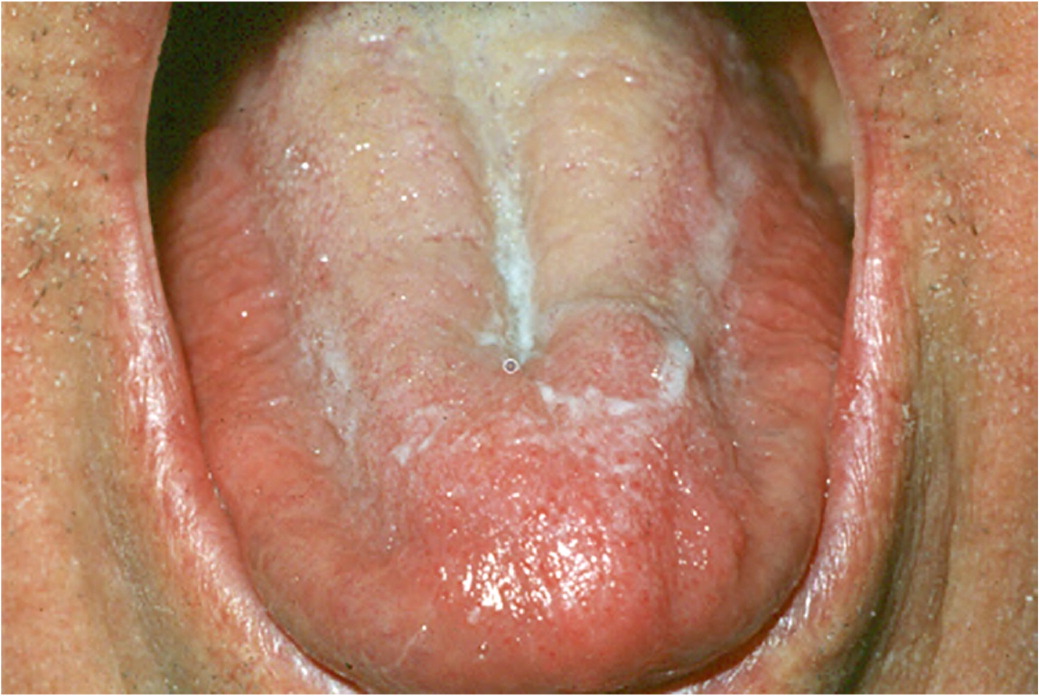
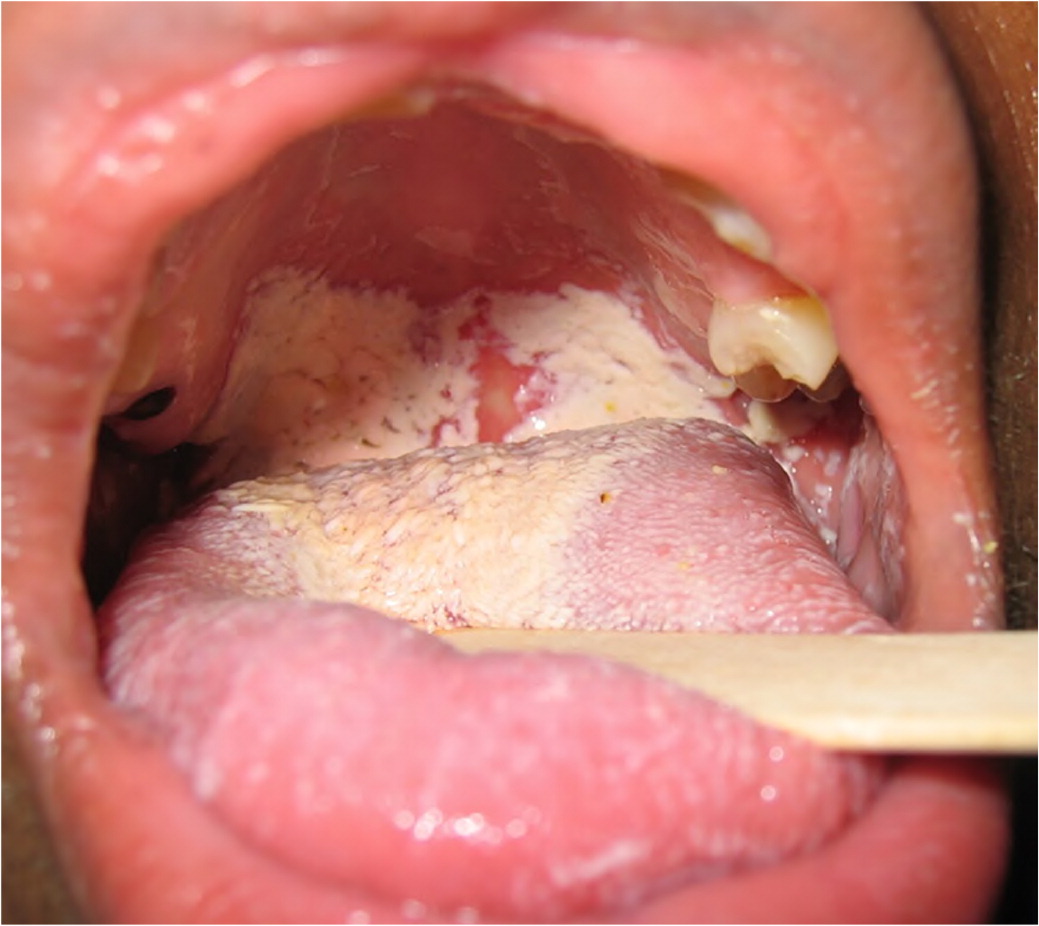
Dry mouth is associated with more than 500 medications.56 The overall prevalence of xerostomia is 10% to 33%, with an incidence of 27% to 32% in the medicated population and 14% to 16% in the nonmedicated population.57 Drug-induced xerostomia occurs most commonly in older people (older than 65 years) and particularly in those patients who are prescribed more than one medication.58 Older age is a major risk factor in the development of xerostomia; however, degradation of salivary flow rates is not an expected process in aging.59
Treatment of xerostomia typically involves symptom control (e.g., sipping water throughout the day, chewing sugarless gum to increase stimulated salivary flow, using artificial salivary substitutes). Physicians can prescribe higher concentration fluoride toothpastes specific for dry mouth to reduce bacterial effects on the teeth or gingiva and to increase patient comfort.60 Sialagogue therapy such as systemic pilocarpine can be prescribed (5 mg four times daily),61–63 although as a parasympathomimetic agonist, it can have worse perceived adverse effects such as sweating or dizziness.64 Cevimeline (30 mg three times daily) might have fewer cholinergic adverse effects and can be used in patients with Sjögren syndrome and other etiologies of dry mouth.65 In general, judicious prescription of medications using the lowest effective dose can aid in prevention of xerostomia in many cases.
Other
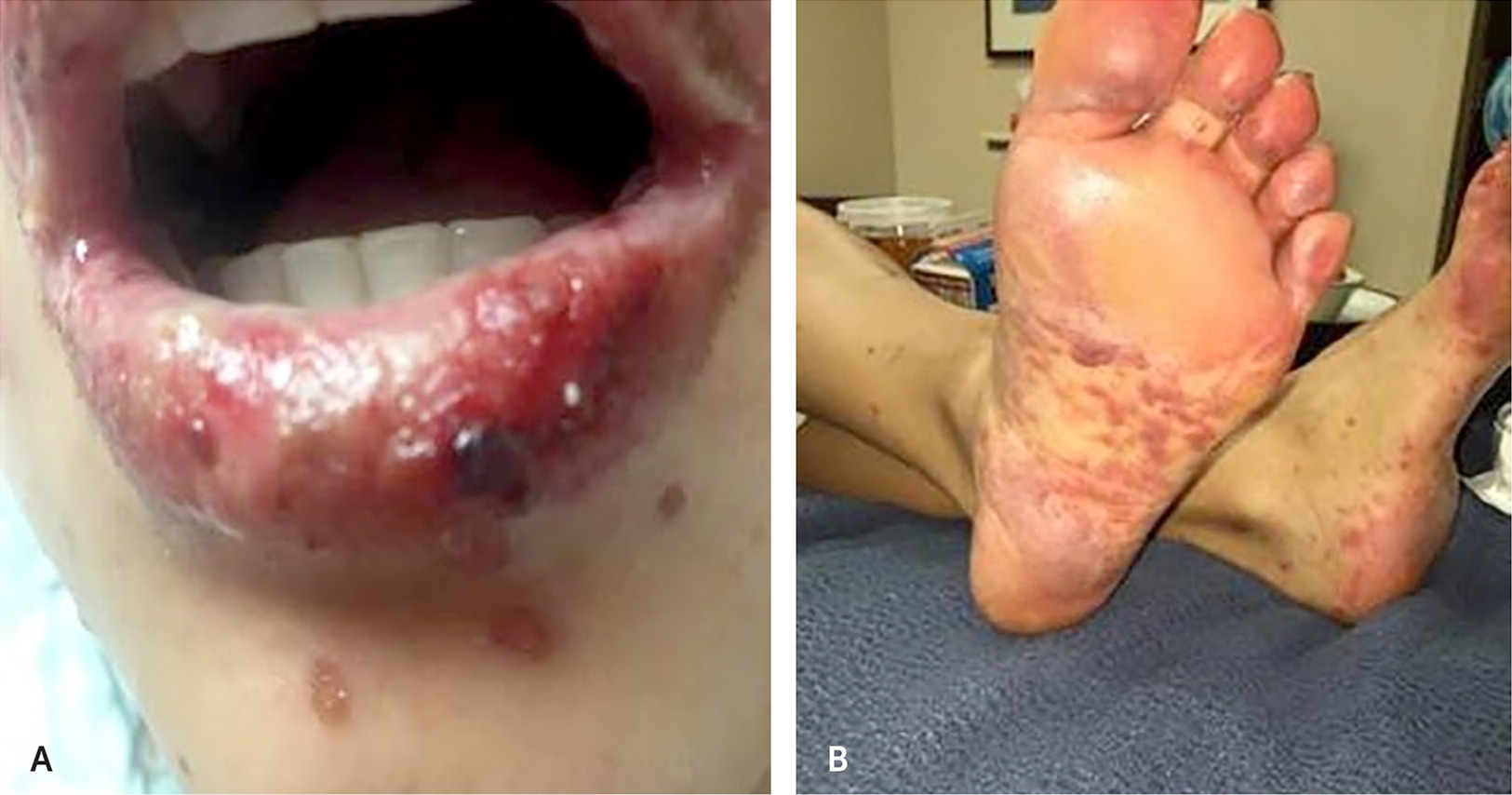
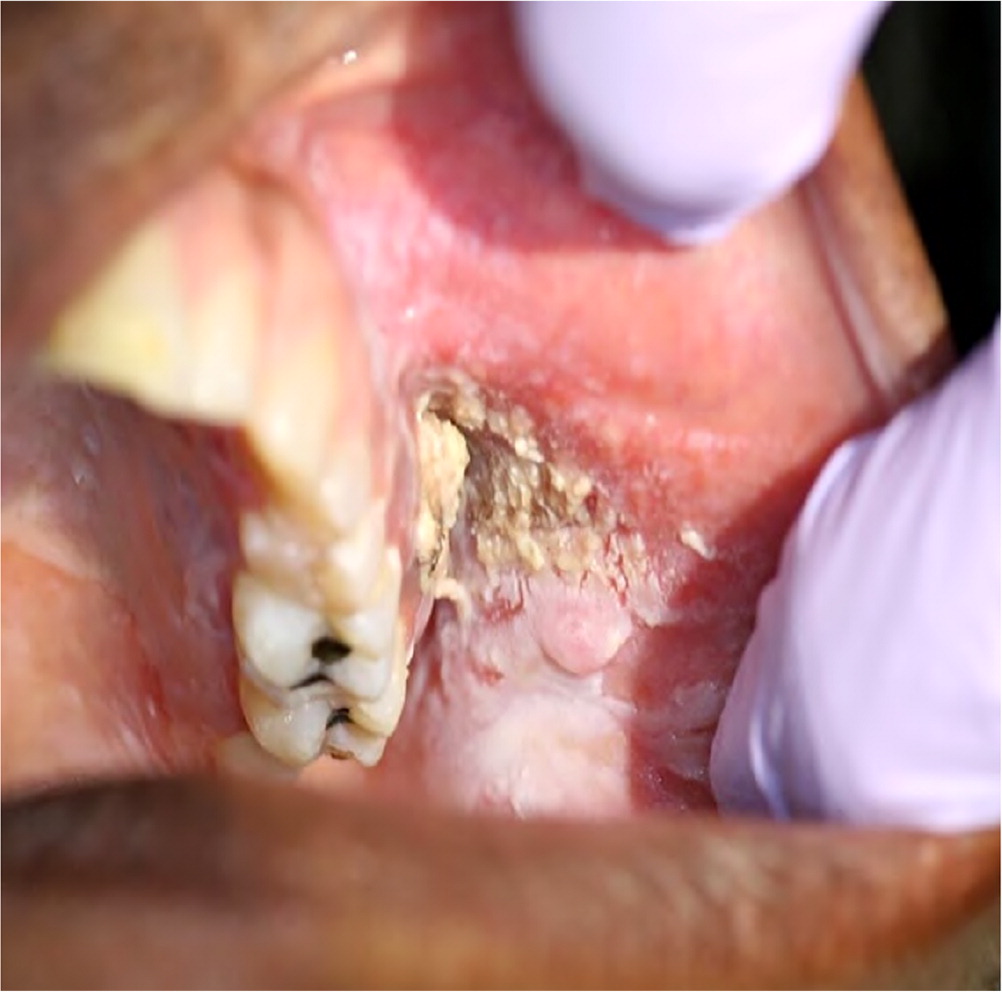
Other oral manifestations include dysgeusia; hypersalivation; numbness; tingling or burning sensations in the mouth; halitosis; hairy tongue; tardive dyskinesia, medication-induced bruxism, or other movement disorder; infection attributable to disturbances in normal oral microbial environment; pemphigus; pemphigoid, salivary gland enlargement; and chemotherapy-induced oral mucositis. In addition to other oral manifestations, misuse of common medications, such as applying crushed aspirin directly to oral mucosa, can cause chemical burns (eFigure A). These oral manifestations may also have other causes, but obtaining an accurate and detailed patient history, determining the onset and duration of symptoms, obtaining an accurate medication list, and knowing the duration of medication use will help clarify whether a prescribed medication is a contributing factor.
Data Sources: A PubMed search was completed using clinical queries with the search terms: gingival hypertrophy: gingival overgrowth, gingival hyperplasia, gingival enlargement, gingival hypertrophy drug-induced plaque, gingival hypertrophy drug-induced substitution; oral hyperpigmentation: hyperpigmentation, gingival pigmentation, medication-induced hyperpigmentation, drug-induced hyperpigmentation, oral pigmentation; oral hypersensitivity reaction: fixed-drug eruption, drug-induced stomatitis, lichenoid stomatitis, Stephen Johnson syndrome [ALDEN]; medication-induced osteonecrosis: drug osteonecrosis jaw, medication-induced osteonecrosis, osteonecrosis dental exam; xerostomia: drug-induced xerostomia, medication-induced salivary gland dysfunction, medication-induced xerostomia, pilocarpine drug-induced xerostomia. All clinical query searches included meta-analyses, randomized controlled trials, clinical trials, and reviews. The Cochrane database, AHRQ's Effective Healthcare Reports, and Trip were also searched. Search date: August 13, 2019.
