
Am Fam Physician. 2021;103(3):177-178
Author disclosure: No relevant financial affiliations.
QuantiFERON-TB Gold+ is a blood test used for the diagnosis of infection with Mycobacterium tuberculosis complex organisms (M. tuberculosis, M. bovis, M. africanum). It is used as an alternative to tuberculin skin testing for latent tuberculosis (TB). QuantiFERON-TB Gold+ is known as an interferon-gamma release assay (IGRA) because M. tuberculosis peptide antigens stimulate the release of interferon-gamma from the T cells of patients with the infection. It is the next generation of QuantiFERON IGRA testing. The previous generation is called QuantiFERON-TB Gold or QuantiFERON-TB Gold In-tube. Results of the blood test may be negative, positive, or indeterminate. A positive test result does not distinguish between active and latent TB.1 The U.S. Preventive Services Task Force recommends testing for latent TB in patients at increased risk of infection.2
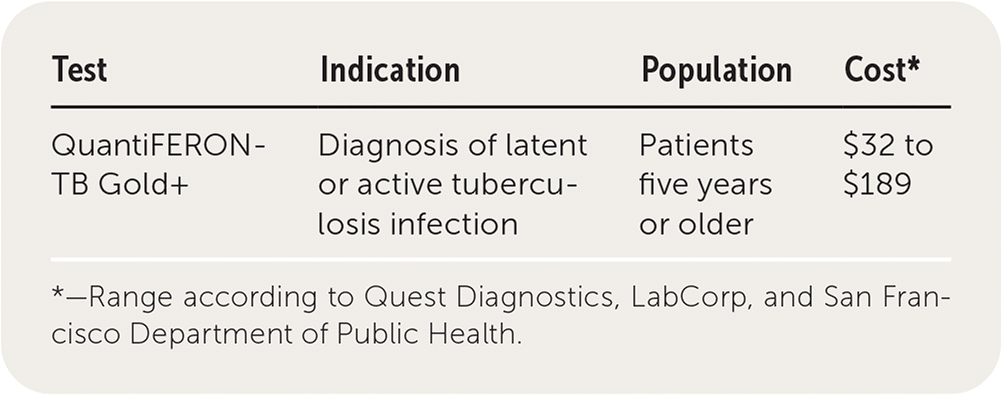
| Test | Indication | Population | Cost* |
|---|---|---|---|
| QuantiFERON-TB Gold+ | Diagnosis of latent or active tuberculosis infection | Patients five years or older | $32 to $189 |
Accuracy
Cross reactivity is known to exist with three nontuberculous mycobacteria: M. kansasii, M. szulgai, and M. marinum. The proteins used to stimulate the cell-mediated response in patients are not found on any other nontuberculous mycobacteria or within the components of the bacille Calmette-Guérin (BCG) vaccine for TB. The manufacturer of QuantiFERON-TB Gold+ conducted seven sensitivity studies and four specificity studies across multiple sites in the United States, Japan, and Australia.1 Table 1 compares the specificity and sensitivity of tuberculin skin testing and the two major IGRA tests, QuantiFERON-TB Gold+ and T-SPOT.TB.1,3–5
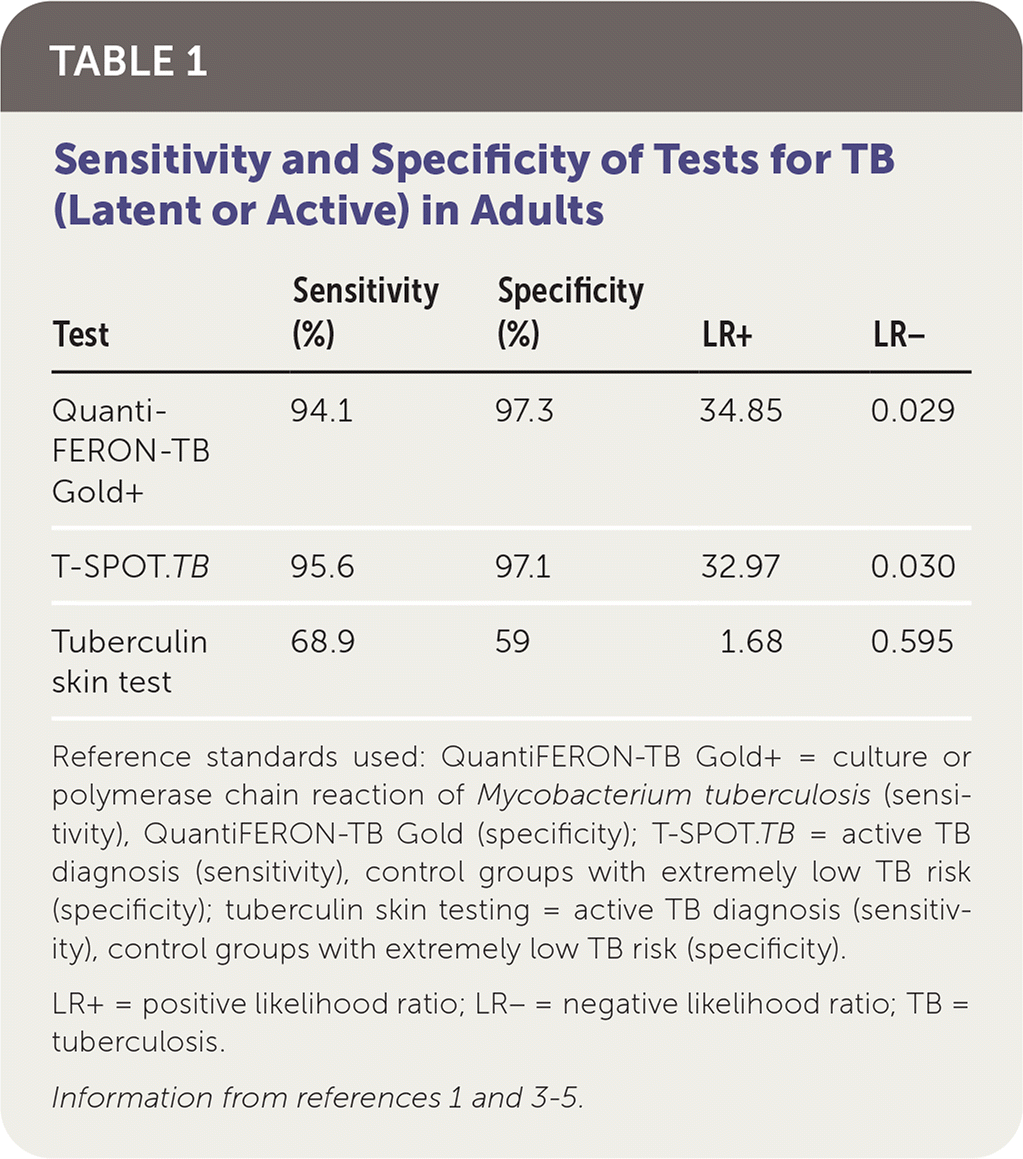
| Test | Sensitivity (%) | Specificity (%) | LR+ | LR− |
|---|---|---|---|---|
| QuantiFERON-TB Gold+ | 94.1 | 97.3 | 34.85 | 0.029 |
| T-SPOT.TB | 95.6 | 97.1 | 32.97 | 0.030 |
| Tuberculin skin test | 68.9 | 59 | 1.68 | 0.595 |
Benefit
QuantiFERON-TB Gold+ testing can be performed during a single visit and does not require a second interpretation visit, as with tuberculin skin testing. The results of QuantiFERON-TB Gold+ testing are objective, do not require training to interpret, and are not subject to variability in reading methods. QuantiFERON-TB Gold+ is more specific than tuberculin skin testing in patients with a history of BCG vaccination, and the BCG vaccine does not affect IGRA results.6
Harms
QuantiFERON-TB Gold+ testing and tuberculin skin testing are unable to distinguish between active and latent TB or predict the risk of progression from latent to active TB. IGRA testing requires a laboratory and is more difficult to perform in resource-limited environments.6 Although indeterminate results for IGRA tests are rare (0% to 2.3% of all results), they may be caused by a compromised immune system or inappropriate handling or testing of blood. Indeterminate results may require retesting or radiography for confirmation, causing a potential delay in diagnosis.1 Because of the higher probability and increased clinical risk of missing latent TB in immunocompromised patients, guidelines recommend performing both IGRA and tuberculin skin tests in these patients and treating for latent infection if either test is positive.7
Cost
QuantiFERON-TB Gold+ costs between $32 and $189.8–10 In one cost-effectiveness study in a correctional facility, tuberculin skin testing cost $18.70 per test compared with $41.97 for QuantiFERON-TB Gold+ testing once all costs were accounted for. However, because of its lower sensitivity, the cost per latent TB infection detected was nearly three times as high with tuberculin skin testing.11 Another study comparing QuantiFERON-TB Gold+ with T-SPOT. TB found that T-SPOT. TB costs about $9 less per test.12
Bottom Line
QuantiFERON-TB Gold+ testing for the diagnosis of active or latent TB is easily interpreted and more specific than tuberculin skin testing in BCG-vaccinated populations. In environments with an easily accessible laboratory, it may be a preferred option for diagnosing latent TB. Joint clinical practice guidelines from the American Thoracic Society, Infectious Diseases Society of America, and the Centers for Disease Control and Prevention recommend IGRA over tuberculin skin testing when latent TB screening is warranted in patients five years or older who are at low to intermediate risk of progression to active TB or who have a history of BCG vaccination.7
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the U.S. Army Medical Department or the U.S. Army at large.
