
Am Fam Physician. 2021;103(8):495-501
Related Putting Prevention into Practice: Screening for Hepatitis B Virus Infection in Adolescents and Adults
As published by the USPSTF.
Summary of Recommendation
The USPSTF recommends screening for hepatitis B virus (HBV) infection in adolescents and adults at increased risk for infection (Table 1). B recommendation.
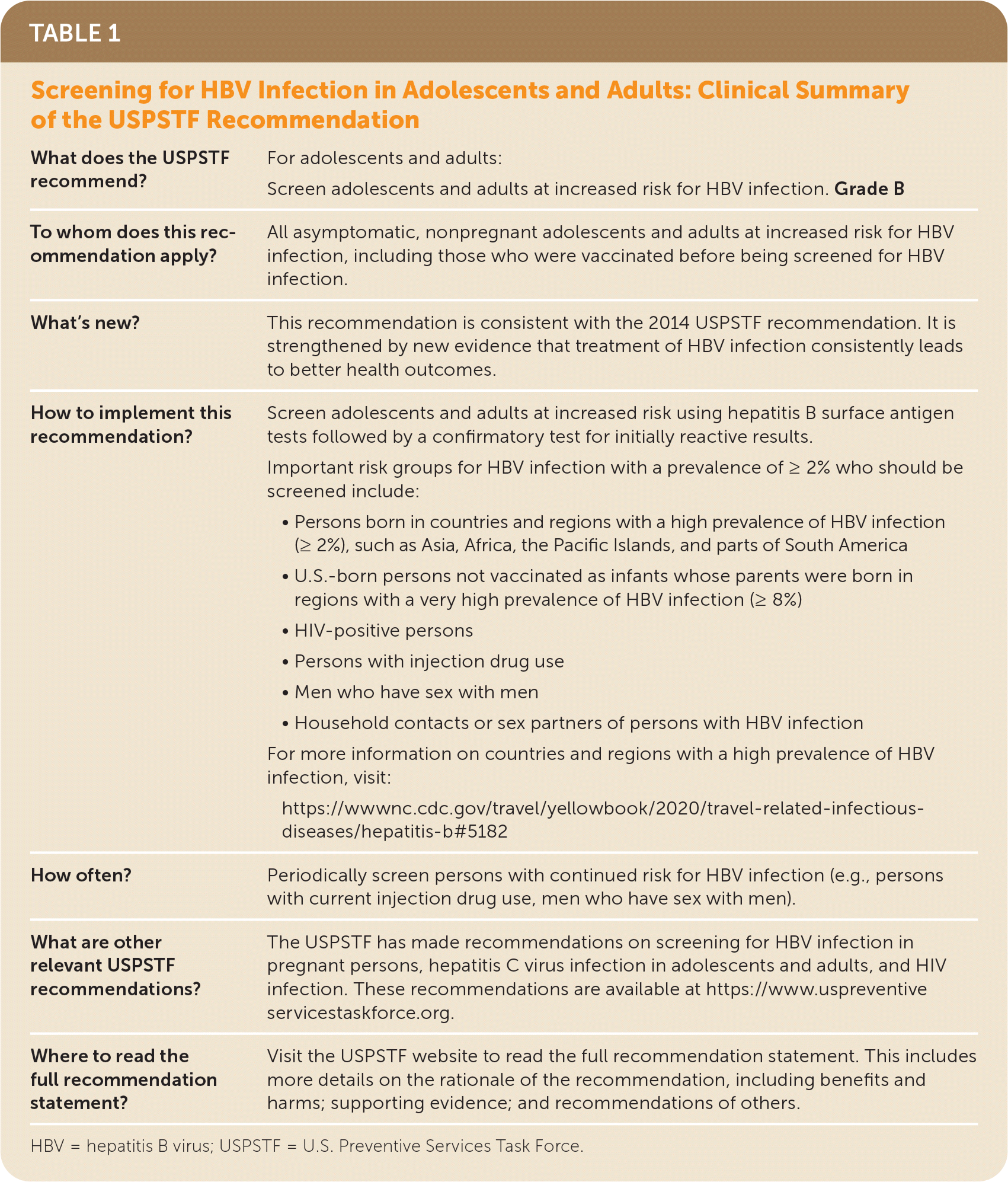
| What does the USPSTF recommend? | For adolescents and adults: Screen adolescents and adults at increased risk for HBV infection. Grade B |
| To whom does this recommendation apply? | All asymptomatic, nonpregnant adolescents and adults at increased risk for HBV infection, including those who were vaccinated before being screened for HBV infection. |
| What's new? | This recommendation is consistent with the 2014 USPSTF recommendation. It is strengthened by new evidence that treatment of HBV infection consistently leads to better health outcomes. |
| How to implement this recommendation? | Screen adolescents and adults at increased risk using hepatitis B surface antigen tests followed by a confirmatory test for initially reactive results. Important risk groups for HBV infection with a prevalence of ≥ 2% who should be screened include:
For more information on countries and regions with a high prevalence of HBV infection, visit: https://wwwnc.cdc.gov/travel/yellowbook/2020/travel-related-infectious-diseases/hepatitis-b#5182 |
| How often? | Periodically screen persons with continued risk for HBV infection (e.g., persons with current injection drug use, men who have sex with men). |
| What are other relevant USPSTF recommendations? | The USPSTF has made recommendations on screening for HBV infection in pregnant persons, hepatitis C virus infection in adolescents and adults, and HIV infection. These recommendations are available at https://www.uspreventiveservicestaskforce.org. |
| Where to read the full recommendation statement? | Visit the USPSTF website to read the full recommendation statement. This includes more details on the rationale of the recommendation, including benefits and harms; supporting evidence; and recommendations of others. |
See the Practice Considerations section for a description of adolescents and adults at increased risk for infection.
Importance
An estimated 862,000 persons in the United States are living with chronic infection with HBV.1 Persons born in regions with a prevalence of HBV infection of 2% or greater, such as countries in Africa and Asia, the Pacific Islands, and parts of South America, often become infected at birth and account for up to 95% of newly reported chronic infections in the United States. Other high-prevalence populations include persons who inject drugs; men who have sex with men; persons with HIV infection; and sex partners, needle-sharing contacts, and household contacts of persons with chronic HBV infection.2
According to the Centers for Disease Control and Prevention (CDC), an estimated 68% of people with chronic hepatitis B are unaware of their infection,3 and many remain asymptomatic until onset of cirrhosis or end-stage liver disease.4,5 This contributes to delays in medical evaluation and treatment and ongoing transmission to sex partners and persons who share objects contaminated with blood or other bodily fluids that contain HBV.3,6 From 15% to 40% of persons with chronic infection develop cirrhosis, hepatocellular carcinoma, or liver failure, which lead to substantial morbidity and mortality.4
USPSTF Assessment of Magnitude of Net Benefit
The USPSTF concludes with moderate certainty that screening for HBV infection in adolescents and adults at increased risk for infection has moderate net benefit.
See Table 2 for more information on the USPSTF recommendation rationale and assessment.
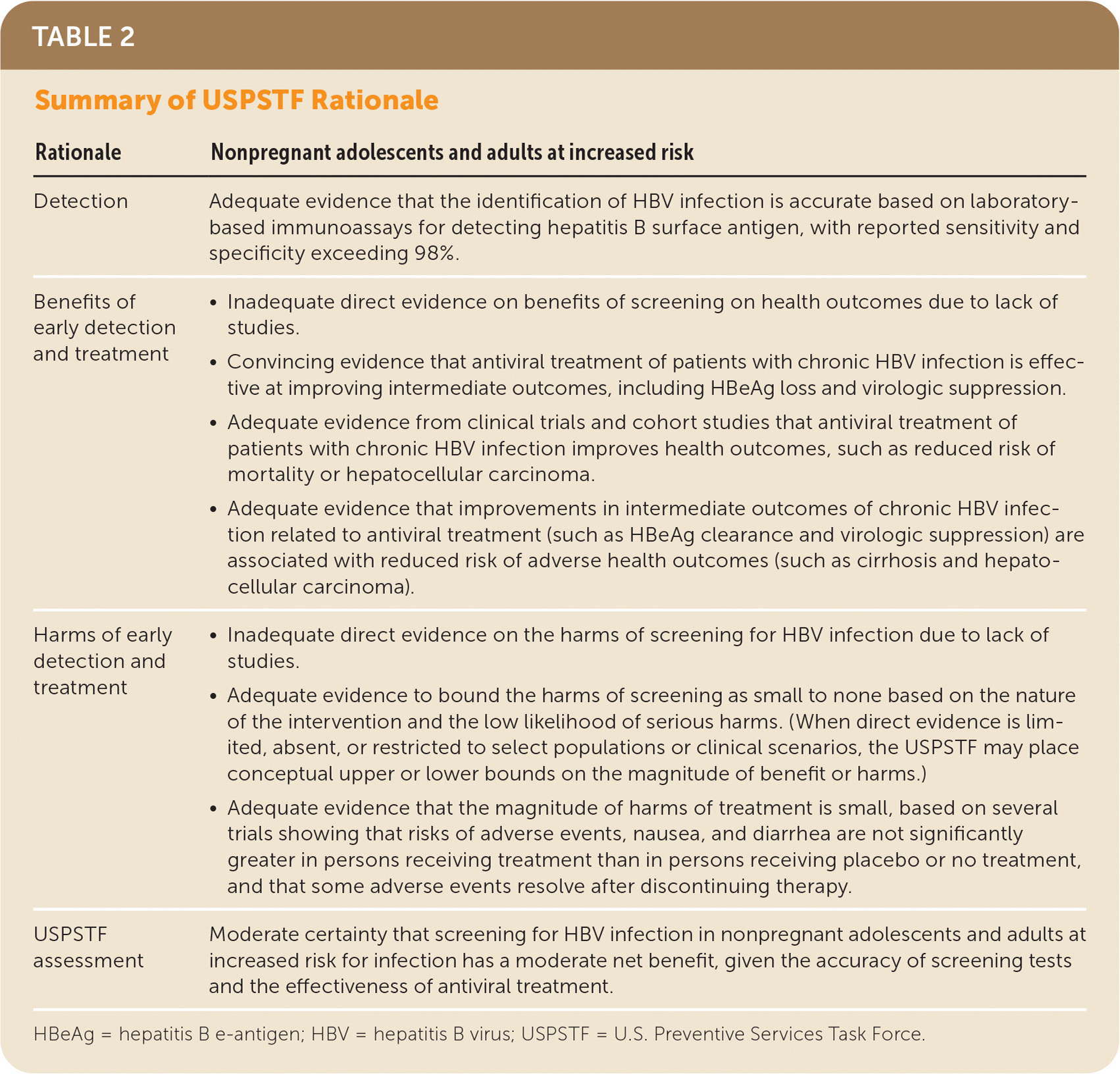
| Rationale | Nonpregnant adolescents and adults at increased risk |
|---|---|
| Detection | Adequate evidence that the identification of HBV infection is accurate based on laboratory-based immunoassays for detecting hepatitis B surface antigen, with reported sensitivity and specificity exceeding 98%. |
| Benefits of early detection and treatment |
|
| Harms of early detection and treatment |
|
| USPSTF assessment | Moderate certainty that screening for HBV infection in nonpregnant adolescents and adults at increased risk for infection has a moderate net benefit, given the accuracy of screening tests and the effectiveness of antiviral treatment. |
For more details on the methods the USPSTF uses to determine the net benefit, see the USPSTF Procedure Manual.7
Practice Considerations
PATIENT POPULATION UNDER CONSIDERATION
This recommendation applies to asymptomatic, nonpregnant adolescents and adults at increased risk for HBV infection, including those who were vaccinated before being screened for HBV infection. The USPSTF has made a separate recommendation on screening in pregnant women.8
ASSESSMENT OF RISK
The risk for HBV infection varies substantially by country of origin in non–U.S.-born persons living in the United States. Persons born in countries with a prevalence of hepatitis B surface antigen (HBsAg) of 2% or greater (Table 3,2,9 Figure10 at https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-b-virus-infection-screening#fig) account for the majority of cases of new chronic HBV infection in the United States; most persons in these countries acquired HBV infection from perinatal transmission.2 Persons born in the United States with parents from regions with higher prevalence are also at increased risk of HBV infection during birth or early childhood, particularly if they do not receive appropriate passive and active immunoprophylaxis (and antiviral therapy for pregnant women with a high viral load) (Figure10 at https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-b-virus-infection-screening#fig).11–13 The CDC classifies HBV endemicity levels by prevalence of positive HBsAg (high [8%], moderate [2%–7%], or low [<2%]) (Figure 10 at https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-b-virus-infection-screening#fig). The estimated prevalence of HBV infection in the general U.S. population is 0.3% to 0.5%,8,9,11,12,14,15 which makes it reasonable to screen adolescents and adults born in countries or regions with an HBsAg prevalence of 2% or greater (regardless of vaccination history in their country of origin) and adolescents and adults born in the United States who did not receive the HBV vaccine as infants and whose parents were born in regions with an HBsAg prevalence of 8% or greater (regardless of their biological mother's HBsAg status).
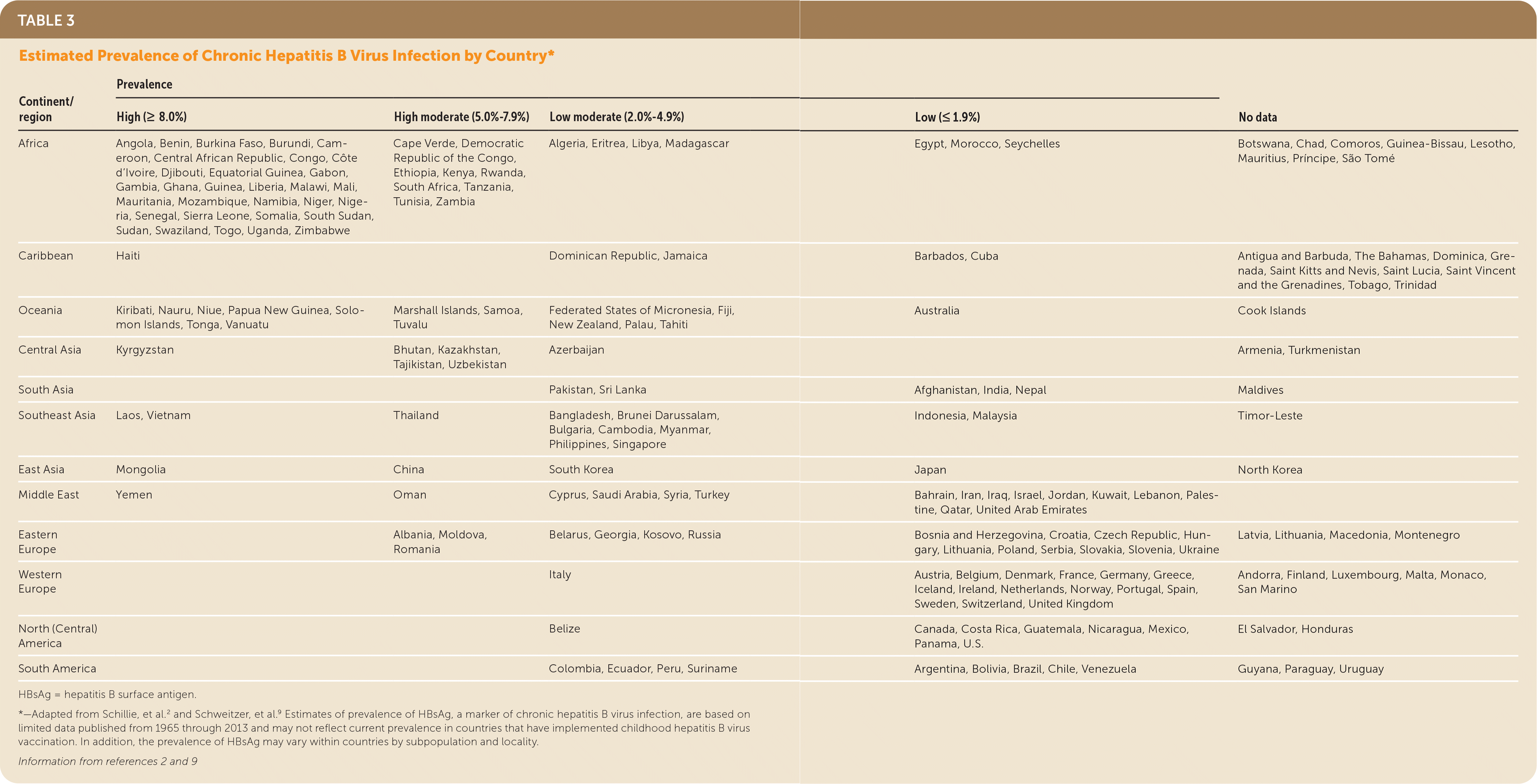
| Continent/region | Prevalence | No data | |||
|---|---|---|---|---|---|
| High (≥ 8.0%) | High moderate (5.0%–7.9%) | Low moderate (2.0%–4.9%) | Low (≤ 1.9%) | ||
| Africa | Angola, Benin, Burkina Faso, Burundi, Cameroon, Central African Republic, Congo, Côte d'Ivoire, Djibouti, Equatorial Guinea, Gabon, Gambia, Ghana, Guinea, Liberia, Malawi, Mali, Mauritania, Mozambique, Namibia, Niger, Nigeria, Senegal, Sierra Leone, Somalia, South Sudan, Sudan, Swaziland, Togo, Uganda, Zimbabwe | Cape Verde, Democratic Republic of the Congo, Ethiopia, Kenya, Rwanda, South Africa, Tanzania, Tunisia, Zambia | Algeria, Eritrea, Libya, Madagascar | Egypt, Morocco, Seychelles | Botswana, Chad, Comoros, Guinea-Bissau, Lesotho, Mauritius, Príncipe, São Tomé |
| Caribbean | Haiti | Dominican Republic, Jamaica | Barbados, Cuba | Antigua and Barbuda, The Bahamas, Dominica, Grenada, Saint Kitts and Nevis, Saint Lucia, Saint Vincent and the Grenadines, Tobago, Trinidad | |
| Oceania | Kiribati, Nauru, Niue, Papua New Guinea, Solomon Islands, Tonga, Vanuatu | Marshall Islands, Samoa, Tuvalu | Federated States of Micronesia, Fiji, New Zealand, Palau, Tahiti | Australia | Cook Islands |
| Central Asia | Kyrgyzstan | Bhutan, Kazakhstan, Tajikistan, Uzbekistan | Azerbaijan | Armenia, Turkmenistan | |
| South Asia | Pakistan, Sri Lanka | Afghanistan, India, Nepal | Maldives | ||
| Southeast Asia | Laos, Vietnam | Thailand | Bangladesh, Brunei Darussalam, Bulgaria, Cambodia, Myanmar, Philippines, Singapore | Indonesia, Malaysia | Timor-Leste |
| East Asia | Mongolia | China | South Korea | Japan | North Korea |
| Middle East | Yemen | Oman | Cyprus, Saudi Arabia, Syria, Turkey | Bahrain, Iran, Iraq, Israel, Jordan, Kuwait, Lebanon, Palestine, Qatar, United Arab Emirates | |
| Eastern Europe | Albania, Moldova, Romania | Belarus, Georgia, Kosovo, Russia | Bosnia and Herzegovina, Croatia, Czech Republic, Hungary, Lithuania, Poland, Serbia, Slovakia, Slovenia, Ukraine | Latvia, Lithuania, Macedonia, Montenegro | |
| Western Europe | Italy | Austria, Belgium, Denmark, France, Germany, Greece, Iceland, Ireland, Netherlands, Norway, Portugal, Spain, Sweden, Switzerland, United Kingdom | Andorra, Finland, Luxembourg, Malta, Monaco, San Marino | ||
| North (Central) America | Belize | Canada, Costa Rica, Guatemala, Nicaragua, Mexico, Panama, U.S. | El Salvador, Honduras | ||
| South America | Colombia, Ecuador, Peru, Suriname | Argentina, Bolivia, Brazil, Chile, Venezuela | Guyana, Paraguay, Uruguay | ||
HBV screening should also be offered to other risk groups defined by clinical and behavioral characteristics in which prevalence of positive HBsAg is 2% or greater. Persons from such risk groups include persons who have injected drugs in the past or currently; men who have sex with men; persons with HIV; and sex partners, needle-sharing contacts, and household contacts of persons known to be HBsAg positive2,3,9,12–14,16,17 (Table 42,9,11,12,15–19). Some persons with combinations of risk factors who are not members of risk factor groups listed previously may also be at increased risk for HBV infection. Clinicians should therefore consider the populations they serve when making screening decisions.
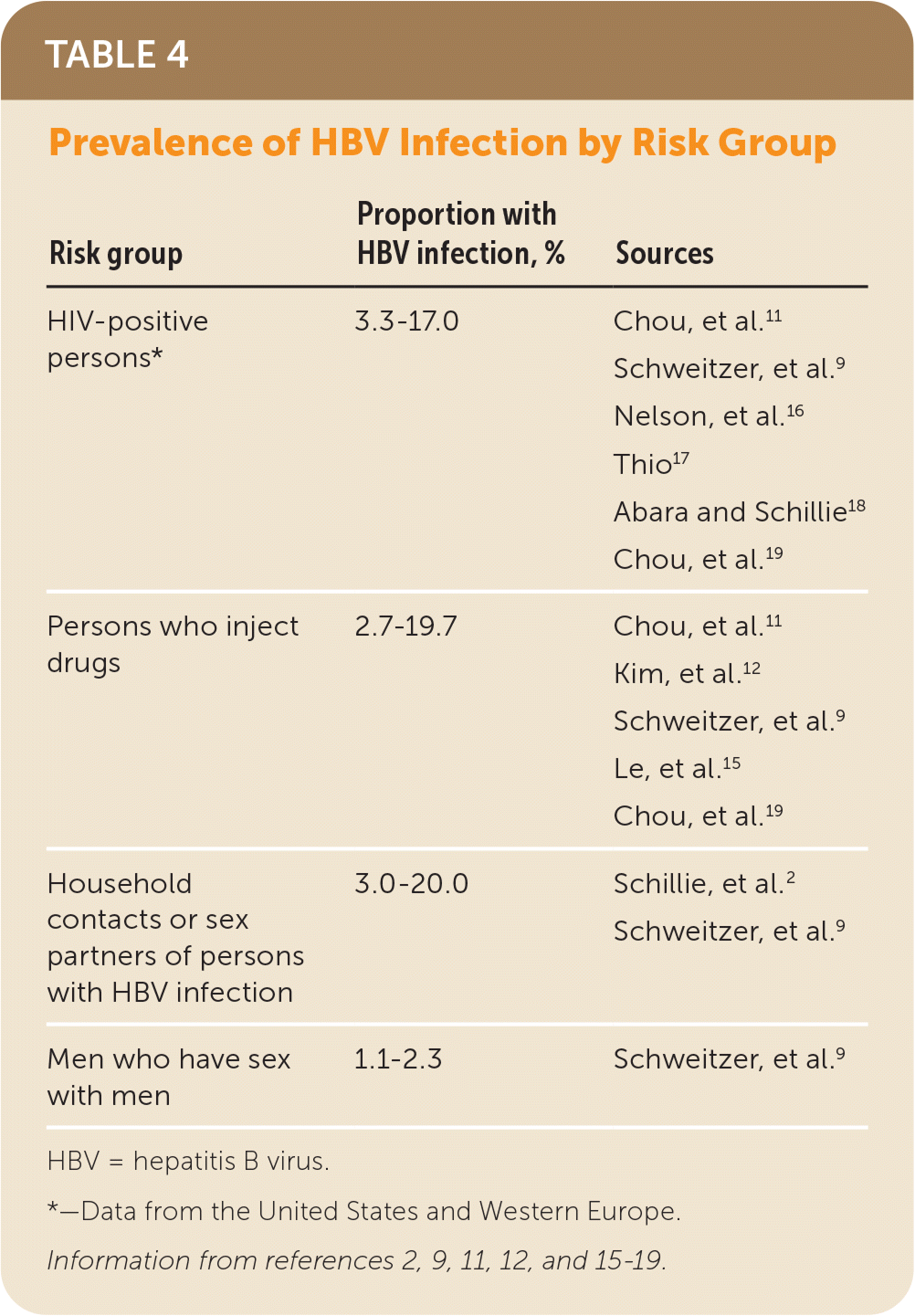
| Risk group | Proportion with HBV infection, % | Sources |
|---|---|---|
| HIV-positive persons* | 3.3–17.0 | Chou, et al.11 |
| Schweitzer, et al.9 | ||
| Nelson, et al.16 | ||
| Thio17 | ||
| Abara and Schillie18 | ||
| Chou, et al.19 | ||
| Persons who inject drugs | 2.7–19.7 | Chou, et al.11 |
| Kim, et al.12 | ||
| Schweitzer, et al.9 | ||
| Le, et al.15 | ||
| Chou, et al.19 | ||
| Household contacts or sex partners of persons with HBV infection | 3.0–20.0 | Schillie, et al.2 |
| Schweitzer, et al.9 | ||
| Men who have sex with men | 1.1–2.3 | Schweitzer, et al.9 |
SCREENING TESTS
A positive HBsAg result indicates chronic or acute infection. Serologic panels performed concurrently with or after HBsAg screening allow for diagnosis and to determine further management. (See the Additional Tools and Resources section for serologic test interpretation.)
SCREENING INTERVALS
For patients with negative HBsAg results who have not received the HBV vaccine series, periodic screening may be useful for those who report continued risk for acquiring HBV transmission, such as persons who continue to inject drugs and men who have sex with men. Clinical judgment should be used to determine screening frequency. The USPSTF found no evidence to determine optimal screening intervals.
TREATMENT
Persons with testing results indicative of acute or chronic HBV infection generally receive education about reducing the risk of transmission (e.g., during childbirth or with sex partners, needle-sharing partners, and household contacts).20 Between 20% and 40% of patients with chronic HBV infection require treatment4 (see Additional Tools and Resources section for information on treatment). Several antiviral medications are approved by the U.S. Food and Drug Administration for treatment of chronic HBV infection.21
IMPLEMENTATION
Many persons at risk for HBV infection are not screened or vaccinated.4 For example, approximately 11% to 67% of non–U.S.-born persons and 28% to 52% of men who have sex with men have undergone HBV screening.4 Low uptake of screening may be related to several barriers, including language, lack of awareness about HBV, limited access to health care, inability to access affordable treatment, stigmatization, concerns about suspension from jobs and other communal activities, and patients' concerns about reporting and follow-up of screening results by public health authorities that may involve notification of close contacts.4,14,22–24 When offering screening, clinicians should understand the positive and negative implications of reporting (as required by most U.S. jurisdictions25), case investigations, and contact notification.24,26
ADDITIONAL TOOLS AND RESOURCES
Several tools may help clinicians implement this screening recommendation. The CDC provides the following tools.
Resources on hepatitis B for professionals (https://www.cdc.gov/hepatitis/hbv/profresourcesb.htm)
A fact sheet on interpretation of hepatitis B serologic tests (https://www.cdc.gov/hepatitis/hbv/pdfs/serologicchartv8.pdf)
Information about HBV prevention, vaccination, transmission, screening, counseling, and treatment (https://www.cdc.gov/hepatitis/HBV/index.htm and https://www.cdc.gov/hepatitis/hbv/hbvfaq.htm)
Information on adolescent and adult HBV vaccination (https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5416a1.htm?s_cid=rr5416a1_e)
OTHER RELATED USPSTF RECOMMENDATIONS
Other related USPSTF recommendations are available at https://www.uspreventiveservicestaskforce.org/uspstf/. These include screening for HBV infection during pregnancy8; screening for hepatitis C virus infection in adults aged 18 to 79 years27; screening for HIV in adolescents and adults aged 15 to 65 years28; and behavioral counseling to prevent sexually transmitted infections.29
This recommendation statement was first published in JAMA. 2020;324(23):2415–2422.
The “Update of Previous USPSTF Recommendation,” “Supporting Evidence,” “Research Needs and Gaps,” and “Recommendations of Others” sections of this recommendation statement are available at https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-b-virus-infection-screening#fullrecommendationstart.
The USPSTF recommendations are independent of the U.S. government. They do not represent the views of the Agency for Healthcare Research and Quality, the U.S. Department of Health and Human Services, or the U.S. Public Health Service.
This summary is one in a series excerpted from the Recommendation Statements released by the USPSTF. These statements address preventive health services for use in primary care clinical settings, including screening tests, counseling, and preventive medications.
The complete version of this statement, including supporting scientific evidence, evidence tables, grading system, members of the USPSTF at the time this recommendation was finalized, and references, is available on the USPSTF website at https://www.uspreventiveservicestaskforce.org/.
