
Am Fam Physician. 2021;103(9):524-525
Author disclosure: No relevant financial affiliations.
Clinical Question
Are ketogenic diets safe and effective at reducing seizure frequency in patients with drug-resistant epilepsy?
Evidence-Based Answer
In children with drug-resistant epilepsy, a ketogenic diet decreases the risk of seizures by 50% after three to four months (absolute risk reduction [ARR] = 37.5%; 95% CI, 19.4% to 67.6%; number needed to treat [NNT] = 3; 95% CI, 1 to 5). (Strength of Recommendation [SOR]: B, based on inconsistent or limited-quality patient-oriented evidence.) Adverse effects such as gastrointestinal symptoms do not occur more often than in children who follow their usual diet. In adults, it is unclear whether ketogenic diets are beneficial, and adverse gastrointestinal effects are common.1 (SOR: B, based on inconsistent or limited-quality patient-oriented evidence.)
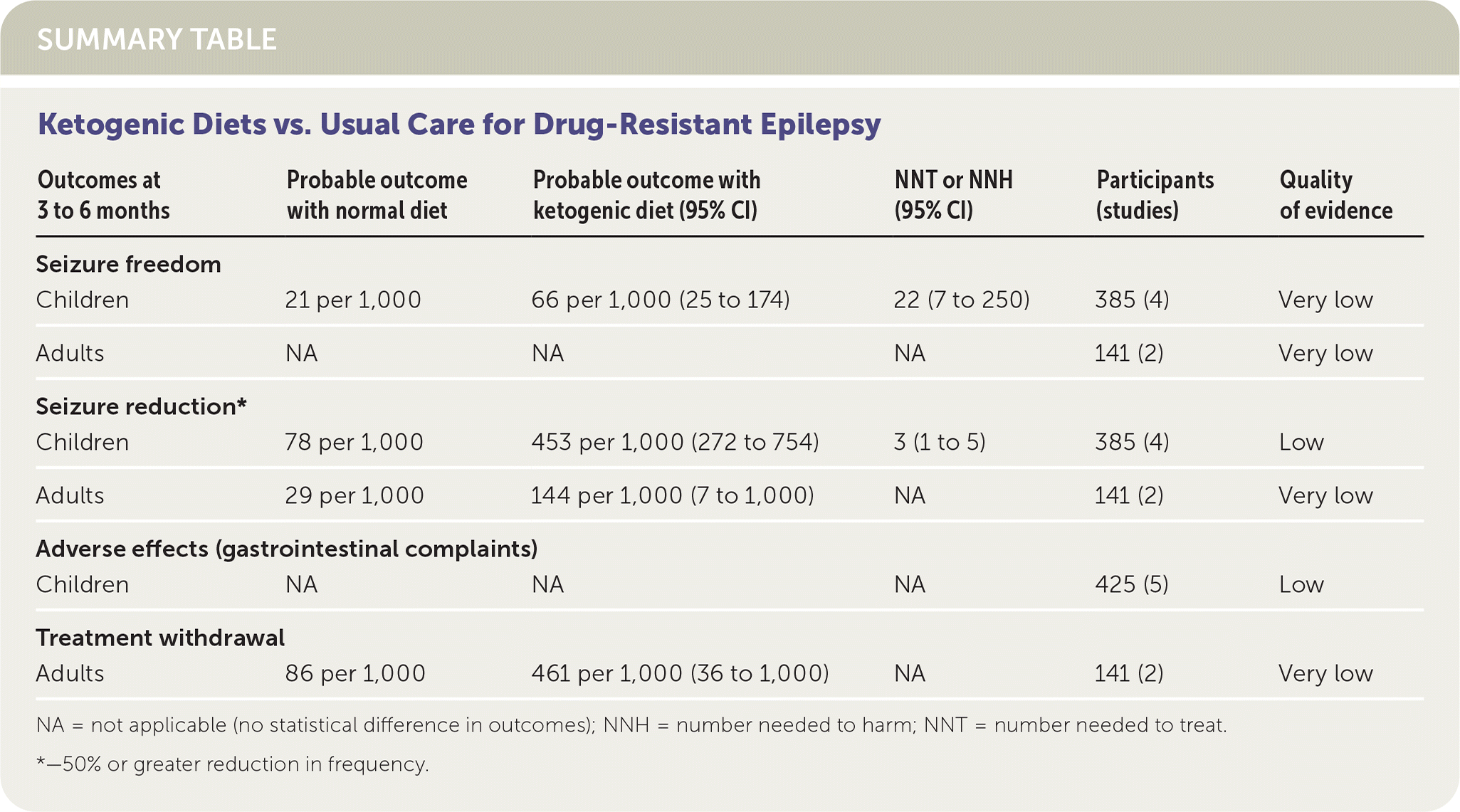
| Outcomes at 3 to 6 months | Probable outcome with normal diet | Probable outcome with ketogenic diet (95% CI) | NNT or NNH (95% CI) | Participants (studies) | Quality of evidence |
|---|---|---|---|---|---|
| Seizure freedom | |||||
| Children | 21 per 1,000 | 66 per 1,000 (25 to 174) | 22 (7 to 250) | 385 (4) | Very low |
| Adults | NA | NA | NA | 141 (2) | Very low |
| Seizure reduction* | |||||
| Children | 78 per 1,000 | 453 per 1,000 (272 to 754) | 3 (1 to 5) | 385 (4) | Low |
| Adults | 29 per 1,000 | 144 per 1,000 (7 to 1,000) | NA | 141 (2) | Very low |
| Adverse effects (gastrointestinal complaints) | |||||
| Children | NA | NA | NA | 425 (5) | Low |
| Treatment withdrawal | |||||
| Adults | 86 per 1,000 | 461 per 1,000 (36 to 1,000) | NA | 141 (2) | Very low |
Practice Pointers
In 2015, approximately 3 million U.S. adults and 470,000 children were diagnosed with epilepsy.2 Worldwide, approximately 30% of people taking two or more antiepileptic medications continue to have seizures; this is termed drug-resistant epilepsy.3 Ketogenic diets have been suggested to reduce seizure frequency in people with epilepsy. Although the exact mechanism is unknown, historically, patients who fasted had less frequent seizures. Ketogenic diets mimic this fasting state by using fat as the primary fuel source. The classic ketogenic diet provides energy in a ratio of four calories of fat to every one calorie of carbohydrate or protein. The authors of this Cochrane review assessed the effectiveness of ketogenic diets, compared with usual diet, at reducing seizure frequency in children and adults with drug-resistant epilepsy.
This Cochrane review included 13 randomized controlled trials (three conducted in the United States, none in Canada; two trials included adults) and 932 participants (221 adults and 711 children).1 The follow-up time ranged from two to 16 months. Results were reported separately for children and adults, and seizure frequency was assessed via self-report. Exclusion criteria in the majority of trials involved persons with known metabolic or neurodegenerative disorders, pregnancy, hyperlipidemia, and renal disease. Primary outcomes were seizure freedom (i.e., being declared seizure-free), seizure reduction (50% or greater reduction in frequency), and adverse effects.
Children who followed a ketogenic diet had higher rates of seizure freedom (ARR = 4.5%; 95% CI, 0.4% to 15.3%; NNT = 22; 95% CI, 7 to 250) and seizure reduction (ARR = 37.5%; 95% CI, 19.4% to 67.6%; NNT = 3; 95% CI, 1 to 5) at three to four months. Adults who followed a ketogenic diet had improved seizure reduction, although the results were not statistically significant. No adult participant experienced seizure freedom.
In children, the most common adverse effects reported were vomiting, constipation, and diarrhea, but these were no more likely with ketogenic diets than with usual diets. Both adult studies demonstrated that these adverse effects were more likely in the ketogenic diet group, but this was also not statistically significant. It is notable that the two adult studies used a modified Atkins diet, which allows for more carbohydrate and protein intake than a traditional ketogenic diet. These studies had conflicting results, with one showing significant decrease in seizure frequency and the other showing no effect.
Limitations of this review include the lack of blinding, which significantly increased performance and detection bias, and small sample size. Given the exclusion criteria, results may not be generalizable.
National Institute for Health and Care Excellence guidelines for management of epilepsy in children recommend referral to a tertiary specialist and consideration of ketogenic diet for those with drug-resistant seizures.4 Family physicians working with patients who have epilepsy, especially children with drug-resistant epilepsy, should be able to discuss the risks and benefits of ketogenic diets with patients.
The practice recommendations in this activity are available at https://www.cochrane.org/CD001903.
Editor's Note: The ARRs, CIs, and NNTs reported in this Cochrane for Clinicians were calculated by the author based on raw data provided in the original Cochrane review.
The opinions herein are those of the author. They do not represent official policy of the Department of Defense or any of its components.
