
Am Fam Physician. 2021;104(1):89-90
Author disclosure: No relevant financial affiliations.
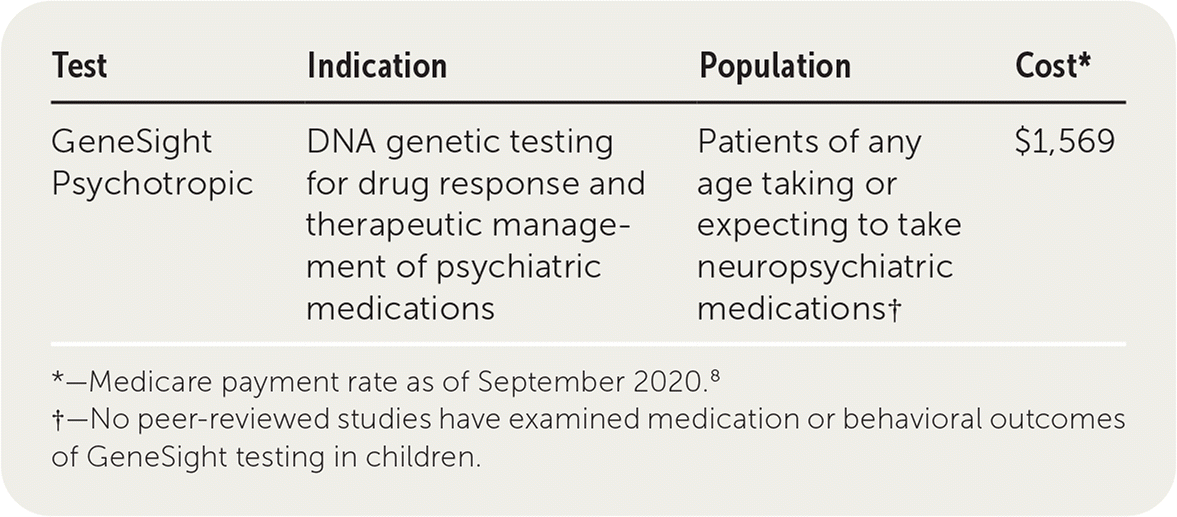
GeneSight Psychotropic is a pharmacogenomic test (multigene panel) that uses a proprietary algorithm to evaluate pharmacokinetic and pharmacodynamic relationships, resulting in drug recommendations or warnings for certain neuropsychiatric medications. Testing can potentially help guide efficient medication selection or dosing. GeneSight has not been submitted to the U.S. Food and Drug Administration (FDA) for approval.
Accuracy
Evaluating a pharmacogenomic test involves analysis of molecular diagnostic test standards. Sensitivity and specificity metrics are not always clear because of the many different aspects of the test, such as enzyme activity, levels of medication in the blood, and metabolizer status. Although some of this information may be helpful in clinical decision-making, it is difficult to calculate accuracy using traditional dichotomous variables.
Test results are summarized in a pharmacogenomics report based on the genes of the patient and possible medication combinations. A color-coded category interpretation is given on the report: green (use as directed), yellow (moderate gene-drug interaction), or red (significant gene-drug interaction). Drugs in the yellow or red category are further labeled with up to nine clinical considerations:
Serum level may be too high, lower doses may be required
Serum level may be too low, higher doses may be required
Difficult to predict dose adjustments because of conflicting variations in metabolism
Genotype may impact drug mechanism of action and result in reduced effectiveness
Use of this drug may increase risk of adverse effects
Serum level may be too low in smokers (only cigarettes)
FDA label identifies a potential gene-drug interaction for this medication
Per FDA label, this medication is contraindicated for this genotype
This medication does not have identified or clinically proven genetic markers that allow it to be categorized
Benefit
GeneSight testing can be performed during a single clinic visit through a blood draw or at home through a buccal swab. A clinician must be enrolled in the GeneSight database to order the test through the company.
Several clinical trials of pharmacogenomic testing have been conducted, but few have been sufficiently powered, randomized, or controlled. The highest-quality and largest trial on pharmacogenomic testing evaluated patients with moderate to severe major depressive disorder and inadequate response to treatment (n = 1,167).4 Patients were randomized to receive the GeneSight test or continue with usual treatment; patients were followed for 24 weeks. At week 8, the predesignated primary end point of the trial, there were statistically higher response and remission rates in the GeneSight group than in the control group (response = 26% vs. 19.9%, number needed to treat = 16; remission = 15.3% vs. 10.1%, number needed to treat = 21). However, after patients and raters were unblinded to the group assignments and GeneSight test results at the end of the study (prescribing clinicians in the guided-care arm always had access to the test results), there were no differences between the two groups in symptom improvement or adverse drug effects.4
Harms
The results of GeneSight testing are subjective, and interpretation of the report is ultimately left to the clinician. Consumer marketing of this test may lead patients to believe that it can predict medication response, when that may not be the case. According to the FDA, only 13 antidepressants have enough pharmacogenomic evidence to warrant changes to therapy, whereas GeneSight evaluates 57 medications.2,5 These medications are metabolized by the enzymes CYP2D6 or CYP2C19, but the GeneSight test looks at 12 different enzymes.2,6 From a drug and enzyme perspective, GeneSight testing may go beyond what is clinically indicated.7 In the United States, an estimated 5% to 10% of patients are poor metabolizers of CYP2D6, and 2% to 15% are poor metabolizers of CYP2C19.6 In the clinical trial discussed above, 28.9% of patients were poor metabolizers of CYP2D6, and 6.7% were poor metabolizers of CYP2C19. This is a higher percentage than a population encountered in clinical practice.4
Cost
Medicare pays $1,569 for the GeneSight test.8 The manufacturer reports that most patients pay between $0 and $330 out of pocket after insurance or financial assistance.9 However, not all insurance companies cover this test, and many require prior authorizations. In general, before the test is approved, a clinician must document the associated diagnosis for which this test is necessary, demonstrated significant adverse effects or at least one medication failure, and why testing would be helpful from a genetic standpoint. This test has limited coverage under the 2021 Centers for Medicare and Medicaid Services' clinical laboratory fee schedule in the pharmacogenomic testing category.10 Although analyses have suggested that pharmacogenomic testing could be cost-effective, these evaluations are based on pooled studies of poor quality, without randomization or blinding.11
Bottom Line
The GeneSight test may assist in drug selection and dosing for individuals having difficulty finding an effective option or who have had intolerable adverse effects after trials of several drugs. However, because only a small population of patients are expected to have genetic phenotypes that would necessitate medication changes, preemptive pharmacogenomic testing for all patients in this population is not cost-effective.12 Routine genetic testing is not recommended. Choosing antidepressants based on health history and symptoms should still be the standard initial approach.
