
Am Fam Physician. 2021;104(2):171-178
Author disclosure: No relevant financial affiliations.
Breast cancer is the leading cause of death from cancer in women worldwide, and the second most common cause of death from cancer in women in the United States. Risk assessment tools can identify the risk of breast cancer, and patients at high risk may be candidates for risk-reducing medications. The choice of medication varies with menopausal status. Breast cancer treatment depends on the stage. Stage 0 is ductal carcinoma in situ, which is noninvasive but progresses to invasive cancer in up to 40% of patients. Ductal carcinoma in situ is treated with lumpectomy and radiation or with mastectomy. If ductal carcinoma in situ is estrogen receptor–positive, patients may also receive endocrine therapy. Early invasive stages (I, IIa, IIb) and locally advanced stages (IIIa, IIIb, IIIc) are nonmetastatic and have three treatment phases. The preoperative phase uses systemic endocrine or immunotherapies when tumors express estrogen, progesterone, or ERBB2 receptors. Preoperative chemotherapy may also be used and is the only option when tumors have none of those three receptors. There are two options for the surgical phase with similar survival rates; a lumpectomy with radiation if the tumor can be excised completely with good cosmetic results, or a mastectomy. Sentinel lymph node biopsy is also performed when there is suspected nodal disease. The postoperative phase includes radiation, endocrine therapy, immunotherapy, and chemotherapy. Postmenopausal women should also be offered postoperative bisphosphonates. Stage IV (metastatic) breast cancer is treatable but not curable. Treatment goals include improving the length and quality of life.
Breast cancer is the second most common cancer diagnosed in women, exceeded only by nonmelanoma skin cancer. It is the leading cause of death from cancer in women worldwide. In the United States, breast cancer is the second most common cause of death from cancer in women, exceeded only by lung cancer, with approximately 316,000 patients diagnosed with breast cancer annually.1,2
| Clinical recommendation | Evidence rating | Comments |
|---|---|---|
| After a histologic diagnosis of breast cancer, all pathology samples should be identified for estrogen, progesterone, or ERBB2 receptor status to direct treatment.24,25 | C | Consensus and expert opinion |
| Sentinel lymph node biopsy is preferred over axillary lymph node dissection for patients without clinical evidence of nodal disease.30 | C | Clinical guideline based on systematic review of randomized controlled trials |
| Patients with advanced breast cancer and metastases to the bones should be offered treatment with denosumab (Prolia) or bisphosphonates such as zoledronic acid (Reclast) or pamidronate (Aredia).24 | C | Consensus and expert opinion |
| For locally recurrent breast cancer initially treated with breast conserving therapy (i.e., lumpectomy plus radiation), further radiation is not recommended; total mastectomy is the standard of care.24 | C | Consensus and expert opinion |

| Recommendation | Sponsoring organization |
|---|---|
| Do not routinely recommend follow-up mammography more often than annually for women who have had radiotherapy following breast-conserving surgery. | American Society for Radiation Oncology |
The treatment of breast cancer requires a multi-disciplinary team of specialists in medical, surgical, and radiation oncology. Because most breast cancers are identified by mammography or by physical examination, primary care physicians are often the first contact for patients with a new breast cancer diagnosis.3 Primary care physicians should be familiar with the diagnostic workup for breast cancer and the basics of treatment.3
Breast Cancer Risk Factors and Risk-Reduction Strategies
Risk factors associated with breast cancer include older age, female sex, early menarche, late menopause, nulliparity, lack of breastfeeding, positive family history, dense breast tissue, hormone therapy, and a history of radiation therapy to the chest.
Genetic variants associated with an increased risk of breast cancer include mutations in breast cancer genes susceptibility 1 and 2 (BRCA1/2) genes.4 Validated risk assessment tools such as the Ontario Family History Assessment Tool or the Seven Questions Family History Screening can help identify women at risk of BRCA1/2 gene mutations or other genetic risks and guide screening and genetic counseling.5
The National Cancer Institute's Breast Cancer Risk Assessment Tool (https://bcrisktool.cancer.gov/) can provide an estimate of breast cancer risk over the next five years, but the tool is not intended to assess risk in patients with BRCA1/2 gene mutations.6 Patients with a greater than 3% risk of breast cancer over the next five years are considered to be at increased risk.
Risk-reducing medications, including the selective estrogen receptor modulators tamoxifen and raloxifene (Evista), or the aromatase inhibitors anastrozole (Arimidex), letrozole (Femara), and exemestane (Aromasin), may be used to treat postmenopausal women 35 years or older who are at increased risk of breast cancer and at low risk of adverse medication effects7 (Table 18–18). In premenopausal women, only tamoxifen should be used for the prevention of primary breast cancer.6
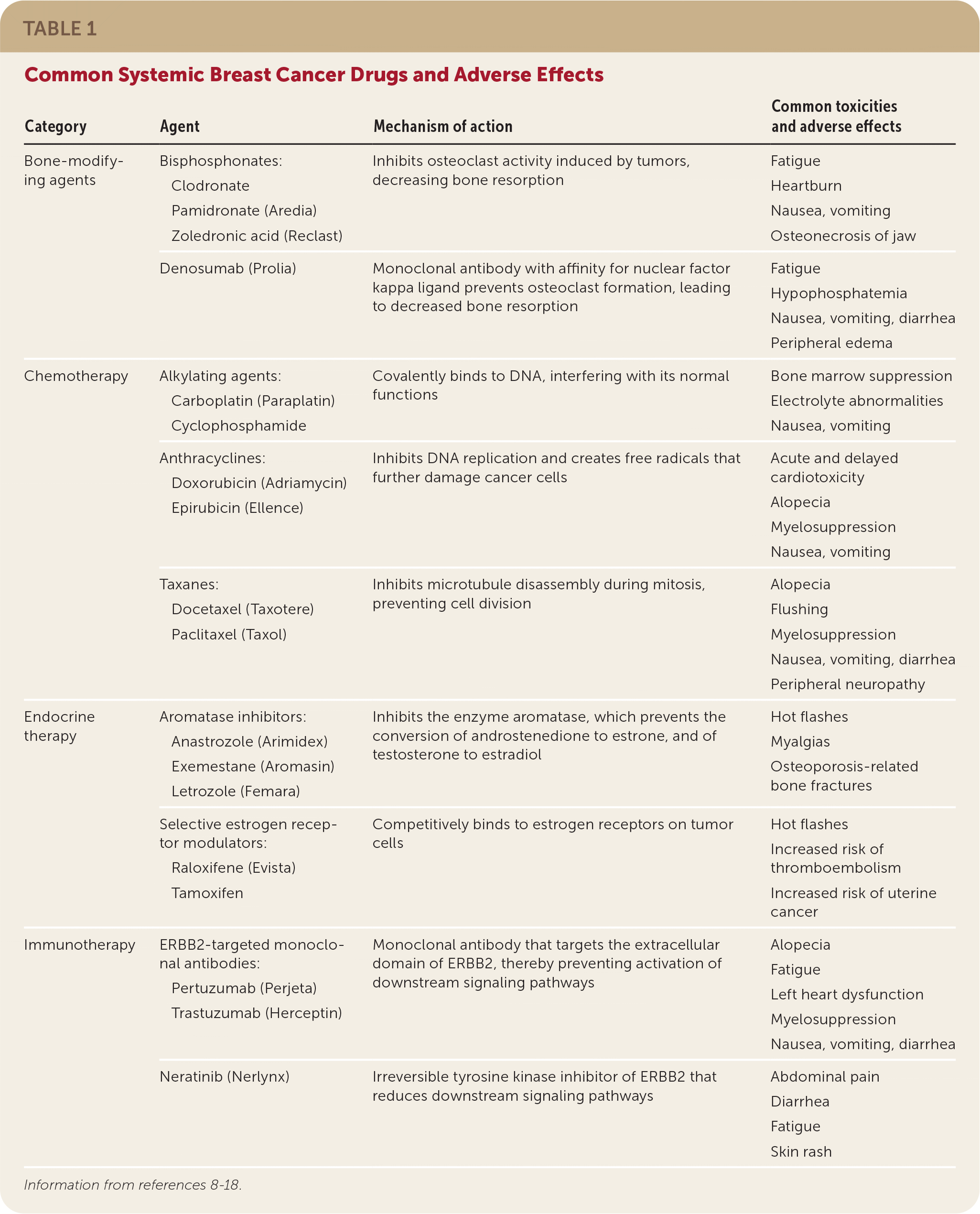
| Category | Agent | Mechanism of action | Common toxicities and adverse effects |
|---|---|---|---|
| Bone-modifying agents | Bisphosphonates: Clodronate Pamidronate (Aredia) Zoledronic acid (Reclast) | Inhibits osteoclast activity induced by tumors, decreasing bone resorption | Fatigue Heartburn Nausea, vomiting Osteonecrosis of jaw |
| Denosumab (Prolia) | Monoclonal antibody with affinity for nuclear factor kappa ligand prevents osteoclast formation, leading to decreased bone resorption | Fatigue Hypophosphatemia Nausea, vomiting, diarrhea Peripheral edema | |
| Chemotherapy | Alkylating agents: Carboplatin (Paraplatin) Cyclophosphamide | Covalently binds to DNA, interfering with its normal functions | Bone marrow suppression Electrolyte abnormalities Nausea, vomiting |
| Anthracyclines: Doxorubicin (Adriamycin) Epirubicin (Ellence) | Inhibits DNA replication and creates free radicals that further damage cancer cells | Acute and delayed cardiotoxicity Alopecia Myelosuppression Nausea, vomiting | |
| Taxanes: Docetaxel (Taxotere) Paclitaxel (Taxol) | Inhibits microtubule disassembly during mitosis, preventing cell division | Alopecia Flushing Myelosuppression Nausea, vomiting, diarrhea Peripheral neuropathy | |
| Endocrine therapy | Aromatase inhibitors: Anastrozole (Arimidex) Exemestane (Aromasin) Letrozole (Femara) | Inhibits the enzyme aromatase, which prevents the conversion of androstenedione to estrone, and of testosterone to estradiol | Hot flashes Myalgias Osteoporosis-related bone fractures |
| Selective estrogen receptor modulators: Raloxifene (Evista) Tamoxifen | Competitively binds to estrogen receptors on tumor cells | Hot flashes Increased risk of thromboembolism Increased risk of uterine cancer | |
| Immunotherapy | ERBB2-targeted monoclonal antibodies: Pertuzumab (Perjeta) Trastuzumab (Herceptin) | Monoclonal antibody that targets the extracellular domain of ERBB2, thereby preventing activation of downstream signaling pathways | Alopecia Fatigue Left heart dysfunction Myelosuppression Nausea, vomiting, diarrhea |
| Neratinib (Nerlynx) | Irreversible tyrosine kinase inhibitor of ERBB2 that reduces downstream signaling pathways | Abdominal pain Diarrhea Fatigue Skin rash |
Bilateral risk-reducing mastectomy may be offered for patients at particularly high risk (e.g., BRCA1/2 gene carriers or carriers of other high-risk, high-penetrance genes).19 There are some data to suggest that bilateral risk-reducing mastectomy results in lower rates of invasive breast cancer and lower mortality rates in high-risk populations.20–22
Breast Cancer Staging and Classification
Breast cancer staging is determined by tumor size, nodal involvement, the presence of metastases, and specific biomarkers such as estrogen receptors, progesterone receptors, and the ERBB2 receptor (formerly HER2).23 After a histologic diagnosis of breast cancer, all pathology samples should be tested for estrogen receptors, progesterone receptors, and ERBB2 status.24,25 Breast cancers that express none of these markers are referred to as triple-negative.
Ductal carcinoma in situ (DCIS) is stage 0, noninvasive breast cancer. Early invasive cancer describes stages I, IIa, and IIb, and locally advanced describes stages IIIa, IIIb, and IIIc. All of these stages of breast cancer are nonmetastatic. Stage IV is metastatic breast cancer.23 Treatments for the various stages of breast cancer are summarized in Table 2.23,24
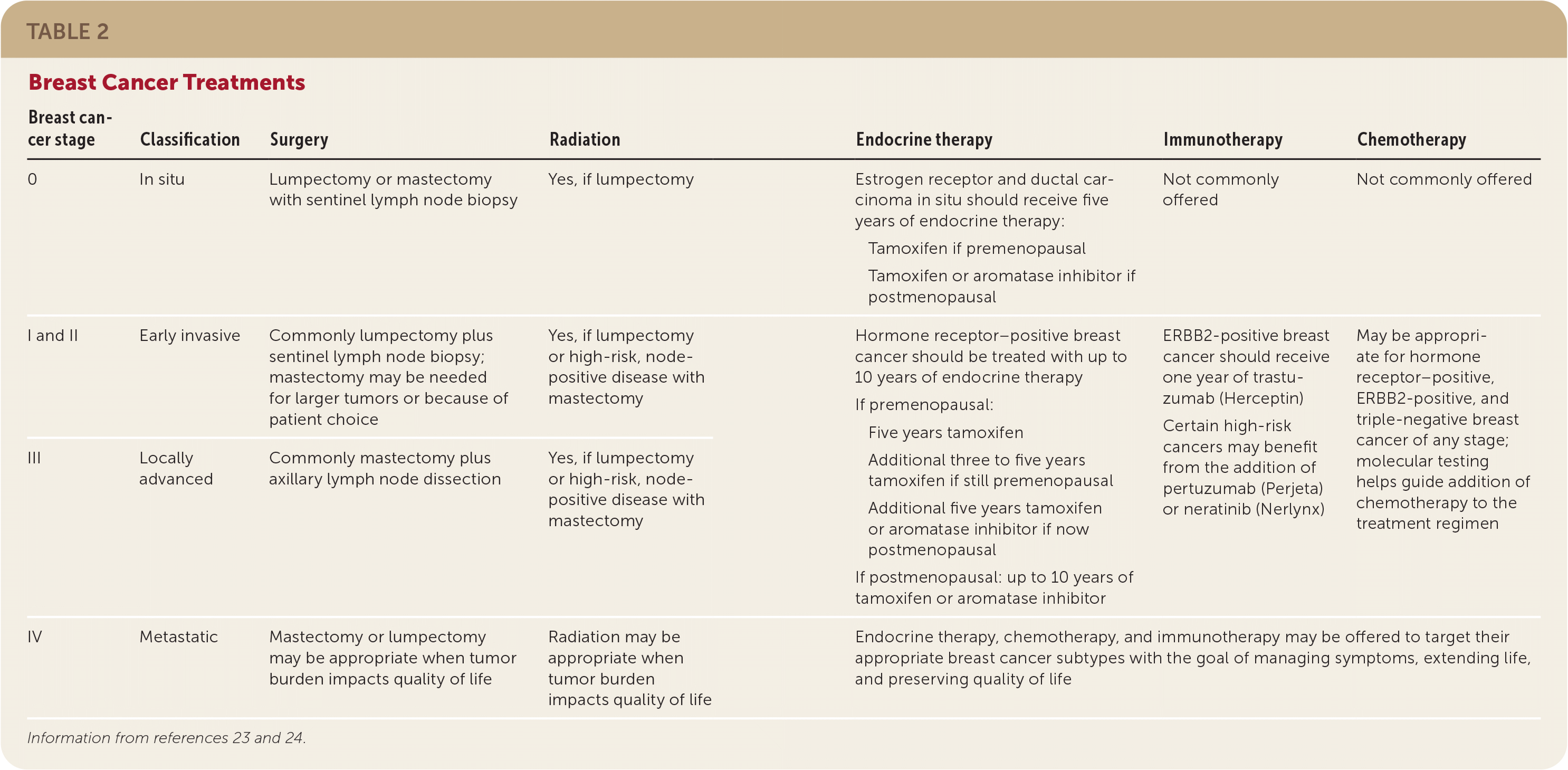
| Breast cancer stage | Classification | Surgery | Radiation | Endocrine therapy | Immunotherapy | Chemotherapy |
|---|---|---|---|---|---|---|
| 0 | In situ | Lumpectomy or mastectomy with sentinel lymph node biopsy | Yes, if lumpectomy | Estrogen receptor and ductal carcinoma in situ should receive five years of endocrine therapy: Tamoxifen if premenopausal Tamoxifen or aromatase inhibitor if postmenopausal | Not commonly offered | Not commonly offered |
| I and II | Early invasive | Commonly lumpectomy plus sentinel lymph node biopsy; mastectomy may be needed for larger tumors or because of patient choice | Yes, if lumpectomy or high-risk, node-positive disease with mastectomy | Hormone receptor–positive breast cancer should be treated with up to 10 years of endocrine therapy If premenopausal: Five years tamoxifen Additional three to five years tamoxifen if still premenopausal Additional five years tamoxifen or aromatase inhibitor if now postmenopausal If postmenopausal: up to 10 years of tamoxifen or aromatase inhibitor | ERBB2-positive breast cancer should receive one year of trastuzumab (Herceptin) Certain high-risk cancers may benefit from the addition of pertuzumab (Perjeta) or neratinib (Nerlynx) | May be appropriate for hormone receptor–positive, ERBB2-positive, and triple-negative breast cancer of any stage; molecular testing helps guide addition of chemotherapy to the treatment regimen |
| III | Locally advanced | Commonly mastectomy plus axillary lymph node dissection | Yes, if lumpectomy or high-risk, node-positive disease with mastectomy | |||
| IV | Metastatic | Mastectomy or lumpectomy may be appropriate when tumor burden impacts quality of life | Radiation may be appropriate when tumor burden impacts quality of life | Endocrine therapy, chemotherapy, and immunotherapy may be offered to target their appropriate breast cancer subtypes with the goal of managing symptoms, extending life, and preserving quality of life | ||
Stage 0, DCIS, Noninvasive Breast Cancer
DCIS is treated with lumpectomy (with a goal of 2-mm surgical margins) and radiation or with mastectomy.28 Sentinel lymph node (SLN) biopsy is done at the time of mastectomy to detect the (unlikely) possibility of lymph node involvement. After mastectomy, an SLN biopsy may not be technically possible.
Radiation therapy is offered to patients having a lumpectomy; the combination of lumpectomy with radiation is considered a breast-conserving therapy. Radiation may be deferred for patients with small, low-grade lesions assessed to have a low risk of recurrence. Radiation is not indicated for patients who are treated with mastectomy.24
Patients with estrogen receptor–positive DCIS and residual breast tissue should receive five years of endocrine therapy. This treatment includes tamoxifen if the patient is premenopausal and tamoxifen or an aromatase inhibitor if postmenopausal.24
Lobular carcinoma in situ was previously considered a malignancy. However, despite its name, it is not a carcinoma and is a proliferative disease that confers an increased risk of future breast cancer. It is no longer included in breast cancer staging guidelines.23
Stages I–III, Early Invasive and Locally Advanced, Nonmetastatic Breast Cancer
Nonmetastatic breast cancer is treated with preoperative and postoperative systemic therapies that include chemotherapy, endocrine therapies, immunotherapy with monoclonal antibodies directed at tumor receptors, and surgery and radiation.
Molecular testing helps guide whether chemotherapy needs to be added to a treatment regimen. For patients with hormone receptor–positive, node-negative breast cancer, including males, a 21-gene expression assay (Oncotype DX) is the preferred assay for disease prognostication and decisions about the addition of chemotherapy.24 When chemotherapy and endocrine therapy are administered postoperatively, chemotherapy always precedes endocrine therapy.24
PREOPERATIVE SYSTEMIC THERAPY
The purpose of preoperative systemic therapy is to decrease the size of resectable breast tumors (allowing for better cosmetic and treatment outcomes), to render unresectable tumors operable, and to allow for SLN biopsy instead of axillary lymph node dissection (ALND) if axillary nodes are no longer detectable. Generally, preoperative therapy is not recommended for early invasive breast cancers (I, IIa, IIb) because tumors are often small enough to undergo resection with lumpectomy.
Preoperative chemotherapy is used for patients with large primary tumors in relation to breast size who want breast-conserving surgery. It is also used for patients with inoperable disease. Chemotherapeutic drugs are the only systemic therapy available for patients with triple-negative breast cancer.24
Preoperative therapy with trastuzumab (Herceptin) and/or pertuzumab (Perjeta), which are monoclonal antibodies directed against ERBB2, is used in addition to chemotherapy when the cancer is ERBB2 positive.
Patients with low-risk estrogen receptor–positive disease, or older adults, may be eligible for preoperative systemic therapy alone, without proceeding to surgery.
A complete response (i.e., the tumor is no longer detectable) to preoperative systemic therapies is associated with favorable outcomes for disease-free survival and overall survival.24 The degree of tumor response to preoperative systemic therapies helps determine the need for and response to any postoperative systemic therapies.
SURGICAL INTERVENTIONS
Lumpectomy with radiation should be offered if negative margins can be achieved with acceptable cosmesis. When the excised tissue is sent to pathology, the specimen's outer surface is marked with ink. A specimen devoid of cancer cells adjacent to the inked surface is considered a negative margin.28
Some tumors may be too large to allow for good cosmesis with lumpectomy and will require a mastectomy. Some women may choose mastectomy over lumpectomy for many other reasons, including family history, gene mutations, peace of mind, or lack of access to health care centers offering radiation therapy.29
In addition to removing the tumor, surgical interventions can also address the potential spread of breast cancer through the lymphatic system. SLN biopsy is done by injecting tracer into the breast and removing the first several axillary nodes into which the tracer drains. An SLN biopsy is preferred over ALND for patients without clinical evidence of nodal disease or with a low nodal burden based on imaging studies.30
The removal of more lymph nodes and adipose tissue of the axilla by ALND is reserved for patients who have positive nodes on SLN biopsy and will have a mastectomy, patients with inflammatory breast cancer (a rare but aggressive cancer that causes redness and swelling of the breast), and patients with positive nodes after preoperative chemotherapy. SLN biopsy does not confer an increased risk of death compared with ALND.31
POSTOPERATIVE THERAPY
Radiation. Radiation therapy is used after surgical excision of breast cancer to eliminate any remaining subclinical disease. It is generally recommended for patients who have had a lumpectomy and for patients with high-risk, node-positive disease who have been treated with mastectomy.4 For patients who have a lumpectomy, radiation decreases the 20-year, ipsilateral breast cancer recurrence rate.32
Partial breast irradiation, which involves treating only the site of lumpectomy and surrounding tissue, requires fewer radiation sessions and decreases acute skin toxicity without increasing the risk of local recurrence.33,34 There is evidence to suggest that whole breast radiation therapy may result in better long-term cosmetic results than partial breast irradiation.34 Shared clinical decision-making for radiation should consider patient life expectancy, tumor response to preoperative systemic therapies, and nodal involvement.35
Endocrine. Approximately two-thirds of breast cancers are hormone receptor–positive (express estrogen receptors, progesterone receptors, or both) and are amenable to treatment with endocrine therapies such as tamoxifen and aromatase inhibitors.36 The mechanism of action and adverse effects for these therapies are summarized in Table 1.8–18
Women who are premenopausal at the time of their hormone receptor–positive breast cancer diagnosis should receive five years of therapy with gonadotropin-releasing hormone (GnRH) for ovarian suppression plus tamoxifen or an aromatase inhibitor.24 Ovarian suppression prevents the ovaries from releasing estrogen, which can lead to accelerated growth for hormone receptor–positive breast cancer. Patients who may benefit more from the GnRH plus aromatase inhibitor regimens are those with hormone receptor–positive, ERBB2-negative breast cancer with a high risk of recurrence.37 After five years of tamoxifen therapy, patients who are premenopausal and originally received tamoxifen receive an additional five years of tamoxifen, and patients who originally received tamoxifen and are now postmenopausal may receive tamoxifen or an aromatase inhibitor for an additional five years. Premenopausal patients who were originally treated with aromatase inhibitors may receive an additional three to five years of aromatase inhibitor therapy.24
Women who are postmenopausal at the time of their breast cancer diagnosis may receive up to 10 years total of endocrine therapy. This may consist of tamoxifen alone, an aromatase inhibitor alone, or a sequence of the two medications. GnRH is not necessary after menopause.
Targeted. Approximately 15% to 20% of breast cancers involve overexpression of ERBB2.38 These cancers have a poor prognosis; however, outcomes are improved with chemotherapy in conjunction with trastuzumab administered every three weeks for one year.38 There is no increase in long-term, disease-free survival with more than one year of therapy.38
Patients with high-risk ERBB2-positive breast cancer determined by larger tumor size and positive nodal status may benefit from additional agents such as pertuzumab, and neratinib (Nerlynx), an oral tyrosine kinase inhibitor that acts on ERBB2-expressing cells. Treatment with therapies in addition to a trastuzumab-containing regimen has demonstrated a small increase in three-year disease-free survival and five-year disease-free survival for pertuzumab and neratinib, respectively.39,40
Chemotherapy. Chemotherapy is used to decrease tumor size before surgery,24 and is administered after surgery to treat breast cancers that express hormone receptors and ERBB2. Chemotherapy is a core treatment for triple-negative breast cancer because endocrine therapy and immunotherapy have no benefit.4,24 Taxane-based, nonanthracycline regimens are used for lower-risk disease, and anthracyclines are included in regimens that target triple-negative cancers with lymph node involvement.41 There are data supporting the use of capecitabine (Xeloda) for triple-negative cancers with lymph node involvement if residual disease is present at the time of surgery.42
Other Treatments. Postmenopausal women who receive postoperative aromatase inhibitor therapy for nonmetastatic breast cancer should be offered bisphosphonate therapy, which appears to decrease the chance of developing bone metastases and fractures and improves survival.24,43,44 Before starting bisphosphonate therapy, patients should undergo a dental examination and should be taking vitamin D and calcium supplements.24
Stage IV, Metastatic Breast Cancer
The median survival rate for patients with metastatic breast cancer has improved over the past several decades with advances in breast cancer therapies. Although metastatic breast cancer is rarely cured, survival is now 24 to 40 months.45
The goals of treatment focus on minimizing symptoms, extending life, and preserving quality of life.4 Endocrine therapy, chemotherapy, and immunotherapy may all be offered to target their respective appropriate breast cancer subtypes. Surgery or radiation following systemic therapy may be appropriate for patients in which tumor burden affects quality of life.46
The 60% to 80% of patients with advanced breast cancer who develop metastases to the bones should be offered treatment with denosumab (Prolia) or bisphosphonates such as zoledronic acid (Reclast) or pamidronate (Aredia).24 These therapies have been shown to decrease the effects of metastases on the bone, such as fractures and hypercalcemia.47
Recurrent Breast Cancer
The treatment of recurrent breast cancer requires a multi-disciplinary approach that considers all potential options for optimal outcomes.24 For locally recurrent breast cancer initially treated with breast-conserving therapy (i.e., lumpectomy plus radiation), additional radiation is not recommended; total mastectomy is the standard of care.24 However, a 2017 study demonstrated promising data that local resection followed by partial breast reirradiation could be an appropriate alternative treatment approach.50
Local recurrence following a mastectomy usually involves the chest wall and requires wide local excision. If the patient did not previously receive radiation, surgical resection followed by radiation therapy is recommended.51
For regional recurrence involving the axillary lymph nodes, surgical resection and radiation therapy are recommended. Radiation therapy without surgery is the standard of care for supraclavicular or internal mammary node recurrence.
The treatment of recurrent disease with distant metastases (i.e., stage IV) is guided by estrogen, progesterone, and ERBB2 receptor status. Treatment algorithms include additional endocrine therapy, chemotherapy, and ERBB2-targeted therapies. Regular assessments with shared decision-making should be implemented to assess ongoing treatment in the setting of palliative and supportive care.
This article updates a previous article on this subject by Maughan, et al.52
Data Sources: A PubMed search was completed in Clinical Queries using the terms breast cancer and treatment. The search included meta-analyses, systematic reviews, clinical trials, and reviews. Also searched were the Cochrane database, U.S. Preventive Services Task Force, DynaMed, American Society of Clinical Oncology Guidelines, Essential Evidence Plus, and UpToDate. Additionally, the search feature on the AAFP website was used with the term breast cancer. Search dates: January 2020, February 2020, and January 2021.
The authors thank Melissa Lazar, MD, and Rebecca Jaslow, MD, for reviewing the manuscript.
